Research - International Journal of Medical Research & Health Sciences ( 2021) Volume 10, Issue 3
Calcitriol Reduces the Contractile Response of Isolated Rabbit Ileum
Hany A El Kattawy1,2* and Shimaa Hadhod12Basic Medical Sciences Department, College of Medicine, Almaarefa University, Saudi Arabia
Hany A El Kattawy, Medical Physiology Department, College of Medicine, Zagazig University, Egypt, Email: hmohammed@mcst.edu.sa
Abstract
Background: 1, 25-dihydroxycholecalciferol [1a, 25(OH)2D3], known as calcitriol, is the active metabolite of vitamin D3 that regulates calcium and phosphate homeostasis. Calcitriol plays a key role in the regulation of immune, cardiovascular and nervous systems. Moreover, it regulates cell proliferation and differentiation with even anti-aging activity. Its action is mediated by the vitamin D receptor found in nearly almost all types of cells providing multiple actions on various tissues. Objectives: This study examined whether calcitriol could change the motility of the isolated rabbit ileum segments and investigate its mechanism of action more thoroughly. Methods: Different serial doses of calcitriol were studied on the isolated ileal smooth muscles of the rabbit in the presence and absence of L-NAME (a nitric oxide synthase inhibitor), Ach, CaCl2, and verapamil (a calcium channel blocker). The ileal smooth muscle contractions were recorded using a force transducer. Results: Calcitriol significantly reduced the spontaneous ileal smooth muscle contractions in a dose-dependent manner. Surprisingly, the incubation of ileal tissue with L-NAME (10-6 M) was able to inhibit the relaxation response induced by calcitriol. Moreover, pretreatment with calcitriol (10-6 M) has reduced the Ach-evoked contractile intestinal response. However, calcitriol failed to modify the verapamilinduced relaxation. Conclusion: Calcitriol reduced the spontaneous contractile response of the isolated rabbit ileum. This relaxation response is induced by calcitriol partly mediated by the nitric oxide pathway with the involvement of calcium channels.
Keywords
Calcitriol, L-NAME, Ileum, Intestinal motility, Rabbit
Introduction
Vitamin D is a biologically inactive fat-soluble compound obtained from sun exposure, animal-based foods, mainly fish oils, and supplements. It is activated through two successive enzymatic hydroxylation steps taking place in the liver and then the kidney. 25-hydroxy-vitamin D3 [25(OH)D3], the most abundant form of vitamin D, is obtained in the liver and used to indicate vitamin D storage and status in the body due to its long half plasma life. Calcitriol, the most physiologically active form of vitamin D3, is obtained from hydroxylation of 25(OH)D3 in the kidney to 1α, 25-dihydroxy-vitamin D3 [1-3]. The intestine is an important target organ for calcitriol effects where it stimulates the dietary intestinal calcium absorption via transcellular and paracellular mechanisms. Calcium enters through specific calcium channels in the brush border membranes. Then, calbindin mediates its intracellular transport where it is actively transported to the blood at the basolateral border via specific carriers [4-6]. Calcitriol actions are mainly mediated by the vitamin D receptor belonging to a subfamily of nuclear receptors. The multiple actions of calcitriol on different tissues may be due to the great expressions of vitamin D receptors in various cell types [7-10]. Briefly, the intestinal smooth muscle contraction is caused by increased cytosolic free calcium ([Ca2+]i) concentrations directly by increased Ca2+ influx through the opening of the membrane voltage-dependent calcium channels or indirectly by Ca2+ release from the intracellular stores through IP3/DAG signaling pathway [11]. The expression of vitamin D receptors in the small intestinal smooth muscles leads us to try to explore a novel action of calcitriol and a possible contribution to intestinal motility regulation [12]. Consequently, this work was conducted to investigate the potential effects of calcitriol on the rabbit small intestinal ileal motility via in vitro tests.
Materials and Methods
Animals and Tissue Preparation
After the approval of the animal research Ethical Committee (IACUC), Zagazig College of Medicine, Egypt, and according to the National Institutes of Health recommendations in the guide for laboratory animals’ care and use, this experimental study was carried out at Zagazig Scientific and Medical Research Center (ZSMRC), College of Medicine. A total of 8 healthy adult rabbits weighing 1850 g-2100 g and aged 11-14 weeks were obtained from the animal house of Zagazig Veterinary Medicine College. The rabbits were kept in temperature-controlled conditions (24 ± 1°C), light cycle (12 h light/dark), and received food and water ad libitum. After acclimatization for one week, overnight fasting rabbit was sacrificed. The abdomen was opened longitudinally and the small intestine was taken out by recognizing the caecum and cut down into two-inch pieces according to the method described by Jabeen, et al. [13]. The isolated ileal strip was thoroughly washed using normal saline. Fatty tissues were dissected and fecal content was removed. Then, it was transferred to the organ bath of 50 ml capacity containing Tyrode’s solution (in mM: NaCl, 136.8 mM; KCl, 2.7 mM; MgCl2, 0.5 mM; CaCl2, 1.3 mM; NaH2PO4, 0.14 mM; NaHCO3, 12.0 mM, Dextrose, 5.5 mM) maintained at 37 ± 0.5°C and continuously aerated with 95% oxygen and 5% carbon dioxide [14]. On termination of the dissected ileal strip was vertically mounted and attached to the bottom of the oxygen tube in a 5 ml tissue organ bath and the other one was linked to a force transducer using surgical silk. The changes in isometric force were recorded by a transducer, amplified, and displayed using LabChart software (Panlab multi-chamber organ bath system, AD instrument, Australia, LapChart serial: 5t8j ff24 798y). The ileal tissue was allowed a period of equilibration of 15 mins and the physiological solution was changed 2 times [15]. The isotonic ileal smooth muscle activity was recorded through the displacement transducer [16].
Chemicals and Reagents
Calcitriol, Ach chloride, L-NAME, verapamil, CaCl2 were purchased from (Sigma Chemical Co., USA). Calcitriol was dissolved in ethanol (0.1% in the organ bath) before dilution with the Physiological Salt Solution (PSS). All the solutions and dilutions were prepared fresh in distilled water at the time of the experiments. Chemicals for the physiologic buffer solution used in this study were purchased from El-Gomhouria Co. for drugs and medical supplies (Egypt). All stock solutions were made and stored following the manufacturer’s guidelines.
Functional Study on the Ileal Contractile Response
A series of in vitro tests was designed to investigate the potential effects of calcitriol on intestinal motility.
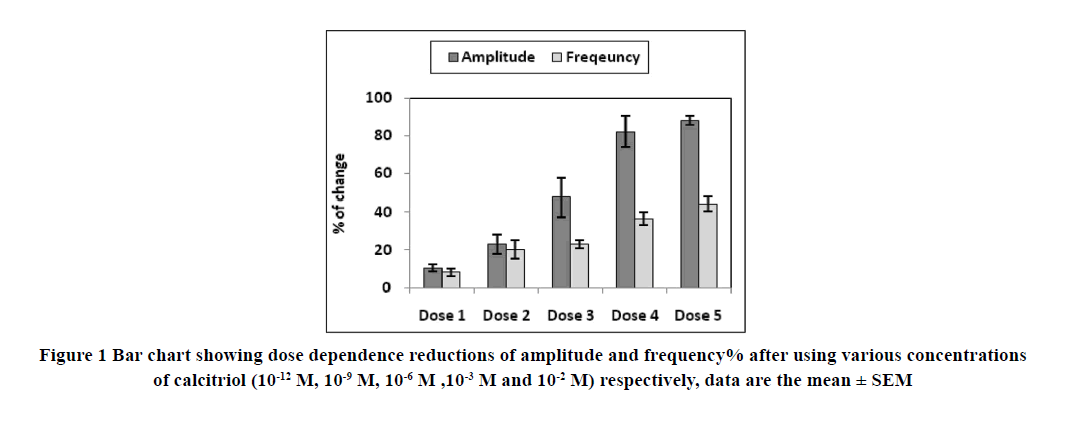
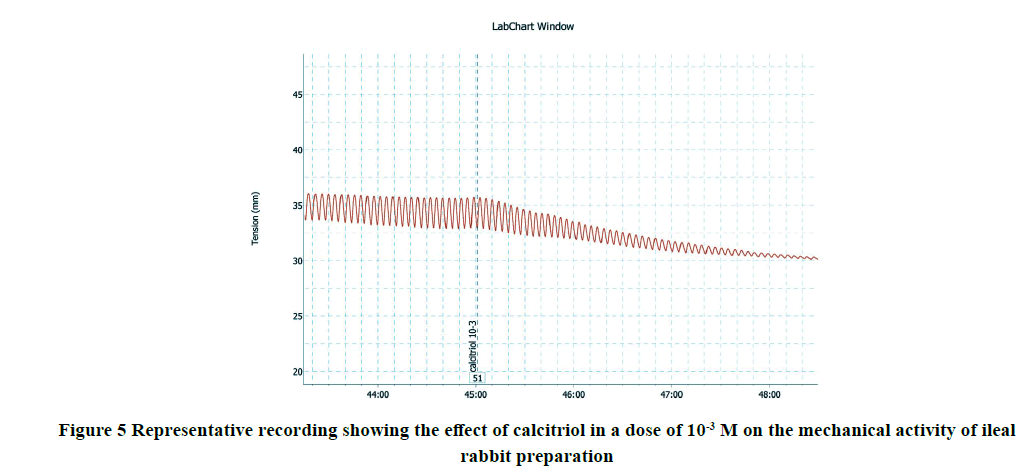
A) The effect of calcitriol on spontaneous contractions of isolated rabbit ileum: Various concentrations of calcitriol (from 10-12 to 10-3 M) was used to find the concentration that produces a 50% inhibition of 100% of the force amplitude (IC50) of the strips. Each dose was applied for 10 min. and then the strips were washed with Tyrode’s solution and the recovery of contractions was monitored. The IC50 was determined and then used throughout the study to detect the effect of calcitriol on the amplitude and frequency of intestinal motility.
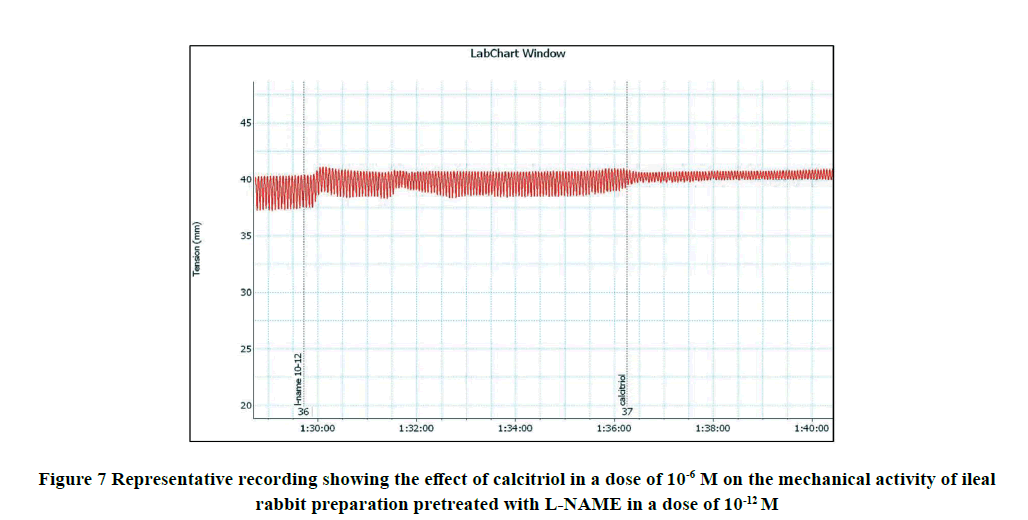
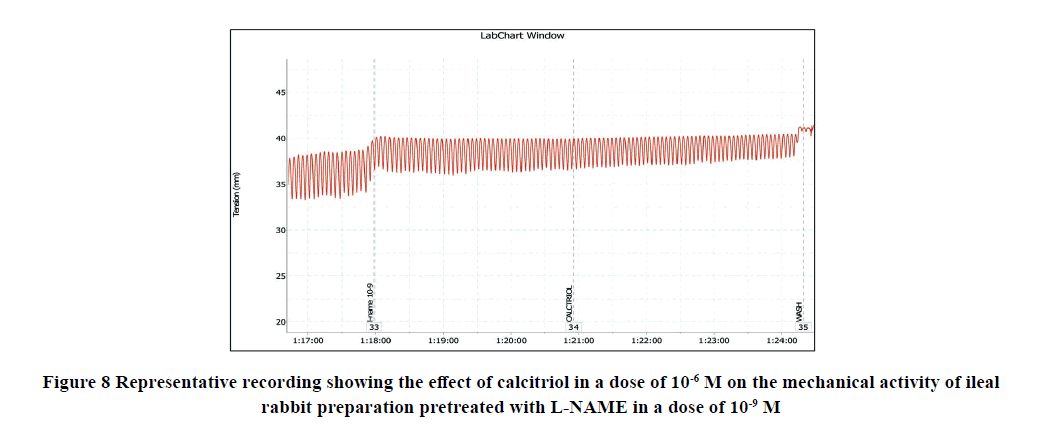
B) Investigation of the involvement of Nitric Oxide Synthase (NOS) in the calcitriol‑induced relaxing response on rabbit isolated ileum: Different doses of L-NAME (from 10-12 to 10-6 M) was applied to the organ bath. After incubation with L-NAME, the amplitude of the calcitriol-induced contractile intestinal response was recorded. Each of them was followed by calcitriol (10-6 M) and then the effect of calcitriol on both amplitude and frequency was recorded.
C) Evaluation of the effect of calcitriol on ileal contractile response induced by Ach: A complete dose-response curve to Ach (10-9 to 10-3 M) was established. At the beginning of each experiment, acetylcholine (Ach, 10-5 M) was applied and served as a control. The first Ach (10-5 M) application on the ileal preparation was applied without calcitriol (taken as a 100% control) and the second application was applied in the presence of calcitriol (10-6 M). After pre-incubation with calcitriol (10-6 M), the amplitude of Ach-evoked contractile intestinal response was recorded. The response obtained was compared with the curve obtained in the absence of calcitriol.
D) Evaluation of the contribution of Ca2+ channels in the mechanism of action of calcitriol:
• The consequence of calcitriol on CaCl2-induced contractions of isolated rabbit ileum: Experiments were also performed using different calcium concentrations from 10-9 M to 10-5 M. The contractile responses of ileal preparations to calcium not pre-treated with calcitriol were recorded. Then, the effect of calcium (10-6 M) on the motility of isolated rabbit ileum pre-treated with calcitriol (10-6 M) was also investigated. The response obtained was compared with the curve obtained in the absence of calcitriol
• The consequence of calcitriol on the motility of isolated ileum pre-treated with Ca2+ channel blocker: Verapamil (Ca2+ channel blocker) was applied to the intestinal strips in different concentrations from 10-9 to 10-4 M and then followed by calcitriol (10-6 M)
Statistical Analysis
The quantitative data are given as the mean ± SEM of 8 different ileal samples. SPSS-24 software (SPSS Inc. Chicago, IL, USA) was used for data analysis. Student t-test and one-way Analysis of Variance (ANOVA) followed by student- Least Significant Differences (LSD) test were used to compare the statistical differences between groups. p-values less than 0.05 values less than 0.05 were considered statistically significant. The means of the parameters before and after exposure to calcitriol during the ileal contractile response were compared. Force amplitude and frequency (the number of contractions in 10 min) are the main parameters used to assess the ileal contractile response. The spontaneous contractile activity for the last 10 min in the control solution was calculated as 100%. The 10-minute calcitriol application was analyzed and expressed as a percentage of the preceding control period.
Results
A) Dose-Dependent Effects of Calcitriol on Spontaneous Contractions of Isolated Rabbit Ileum
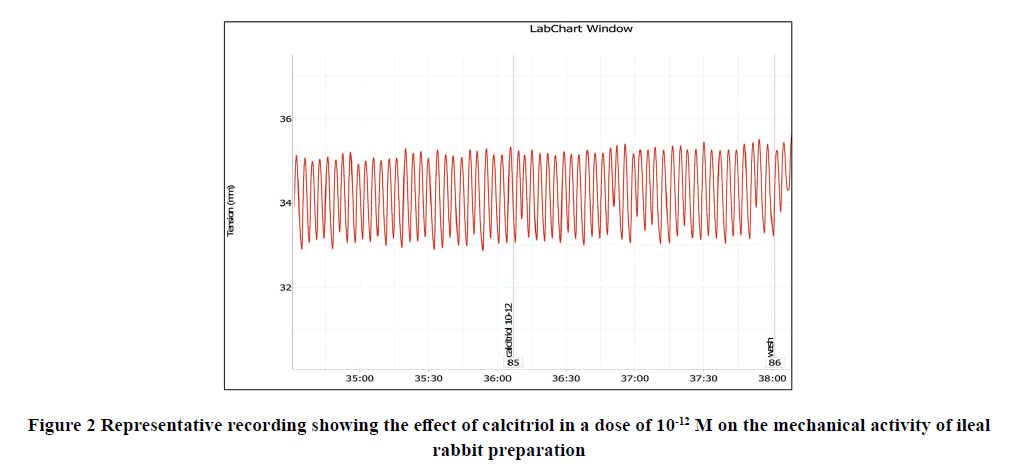
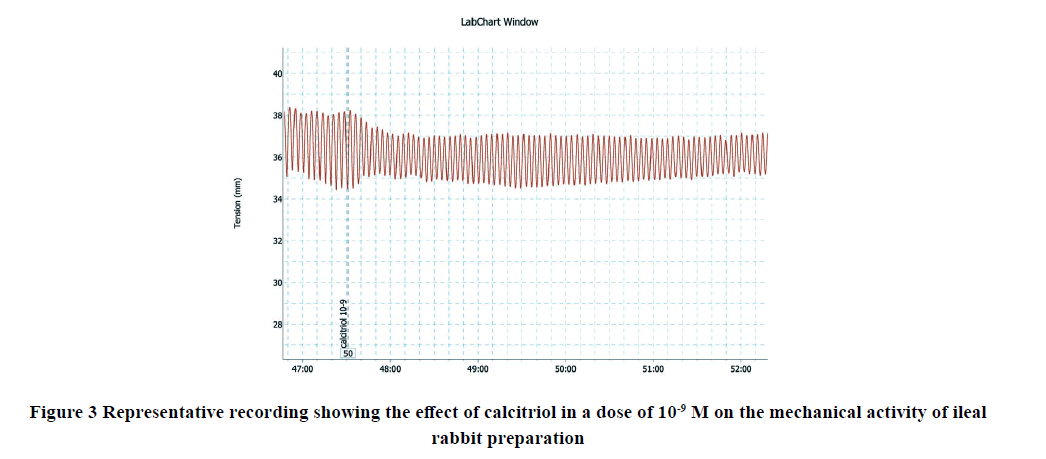
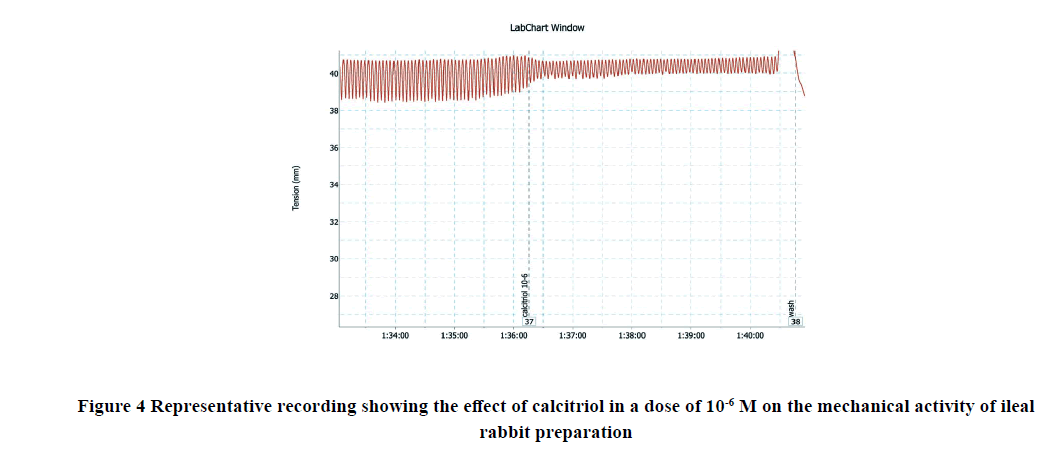
Applications of calcitriol progressively and significantly inhibited the amplitude and frequency of spontaneous intestinal contractions of isolated rabbit ileum in a dose-dependent manner as shown in Table 1 and Figures 1-7. Application of calcitriol (10-6 M) significantly decreased the amplitude of spontaneous intestinal contraction to 47.6 ± 10.3% and frequency to 23.125 ± 1.9%. However, administrations of calcitriol in doses of 10-3 M and 10-2 M significantly reduced the amplitude of spontaneous intestinal contractility to 82.2 ± 8.2% and 88.2 ± 2.3% respectively, and the frequency to 36.7 ± 3.4 and 44.2 ± 3.68 respectively. Therefore, the calcitriol concentration of 10-6 M was taken as the IC50 and this concentration was used throughout the study.
| Calcitriol (M) | Amplitude | Frequency |
| 0/(control) | 1 00.0000 | 1 00.0000 |
| 10-12 M | 10.5 ± 1.9* | 8.28 ± 1.9* |
| 10-9 M | 23.1 ± 5.2* | 20.17 ± 4.8* |
| 10-6 M | 47.6 ± 10.3** | 23.1 ± 1.9** |
| 10-3 M | 82.1 ± 8.1*** | 36.7 ± 3.4*** |
| 10-2 M | 088.2 ± 2.3*** | 44.17 ± 3.6*** |
The values represent the mean and SEM, respectively % (n=8)
*p<0.05 compared to the control; **p<0.01 compared to the control; ***p<0.001 compared to the control.
B) The Influence of Calcitriol on the Motility of Isolated Ileum Pre-Treated with L-NAME
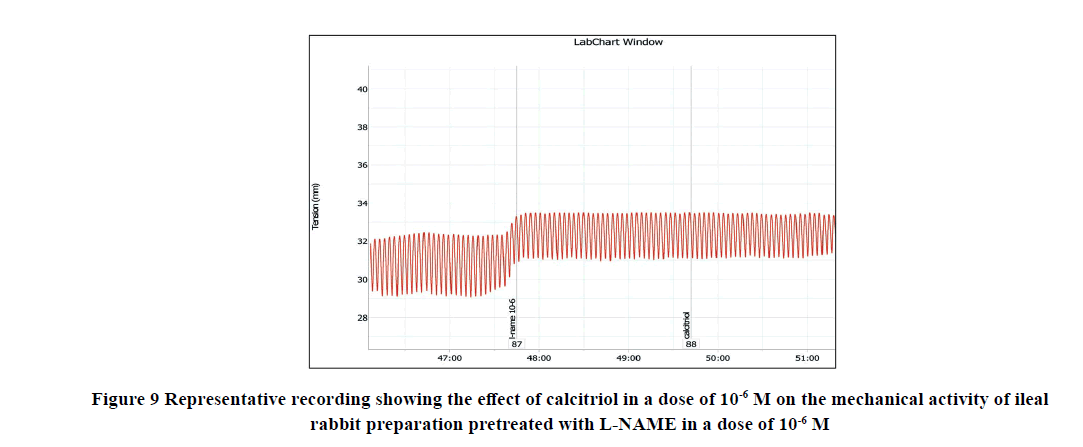
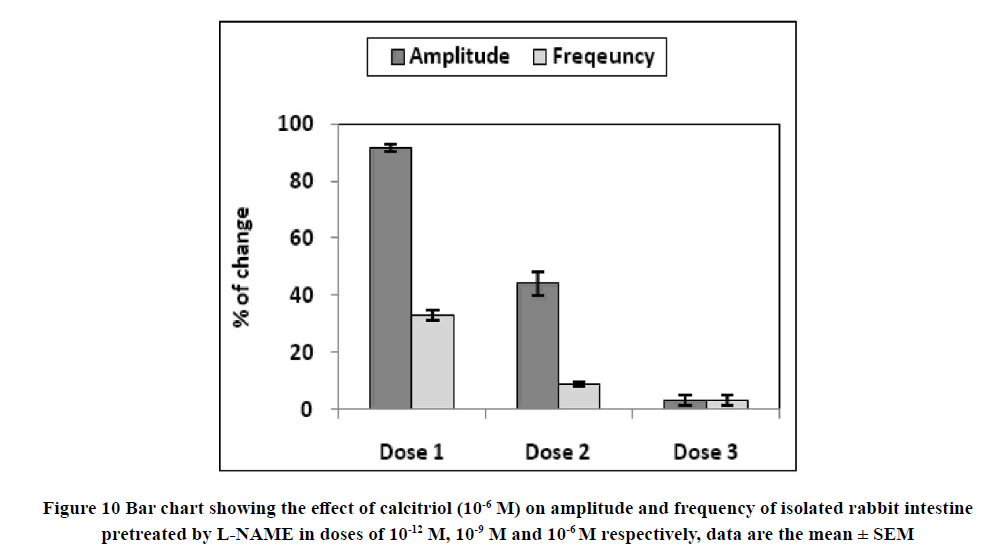
Application of calcitriol (10-6 M) after serial doses of L-NAME from 10-12 M to 10-9 M induced a significant decrease in the amplitude of the spontaneous intestinal contraction to 91.4 ± 1.3% and 44.16 ± 4.1% respectively and the frequency to 32.9 ± 1.8% and 8.97 ± 0.87% respectively. However, when calcitriol (10-6 M) was applied after L-NAME (10-6 M), it had no significant effect on the amplitude neither the frequency of spontaneous intestinal contractility. Therefore, L-NAME in a dose of 10-6 M was used to block the relaxant effect of calcitriol on the isolated rabbit ileum as shown in Table 2 and Figures 8-11.
| Calcitriol (M) | Amplitude | Frequency |
|---|---|---|
| 0/(control) | 1 00.0000 | 1 00.0000 |
| 10-12 M | 2.11 ± 0.1*** | 7.75 ± 0.4*** |
| 10-9 M | 1.5 ± 0.2** | 0.9 ± 0.2** |
| 10-6 M | 0.04 ± 0.01 NS | 0.4 ± 0.1 NS |
The values represent the mean and SEM, respectively % (n=8).
**p<0.001 compared to the control; ***p<0.001 compared to the control; NS=p>0.05
C) The Influence of Calcitriol on Ach-Induced Contractions on Isolated Rabbit Ileum
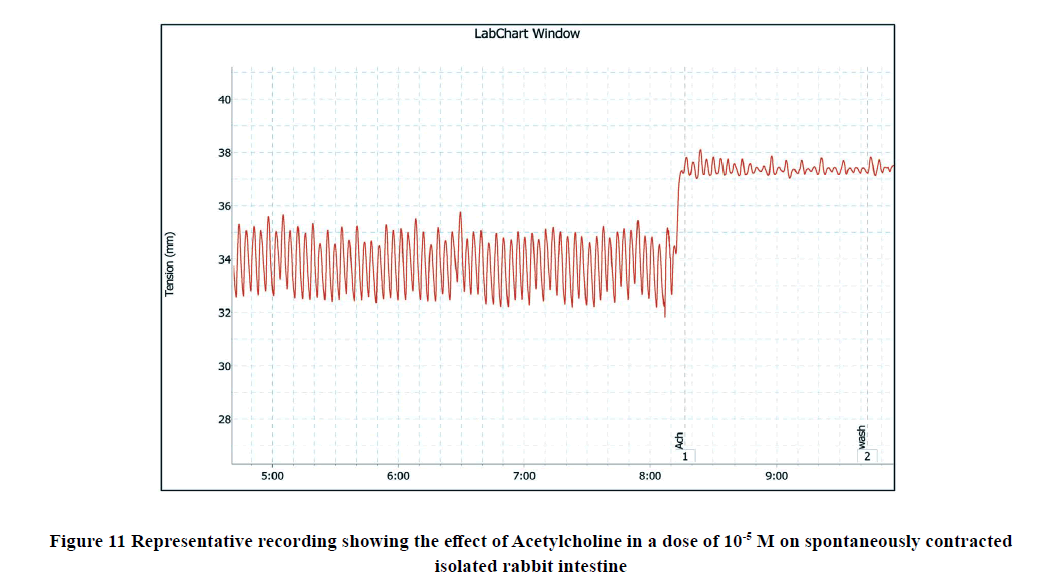
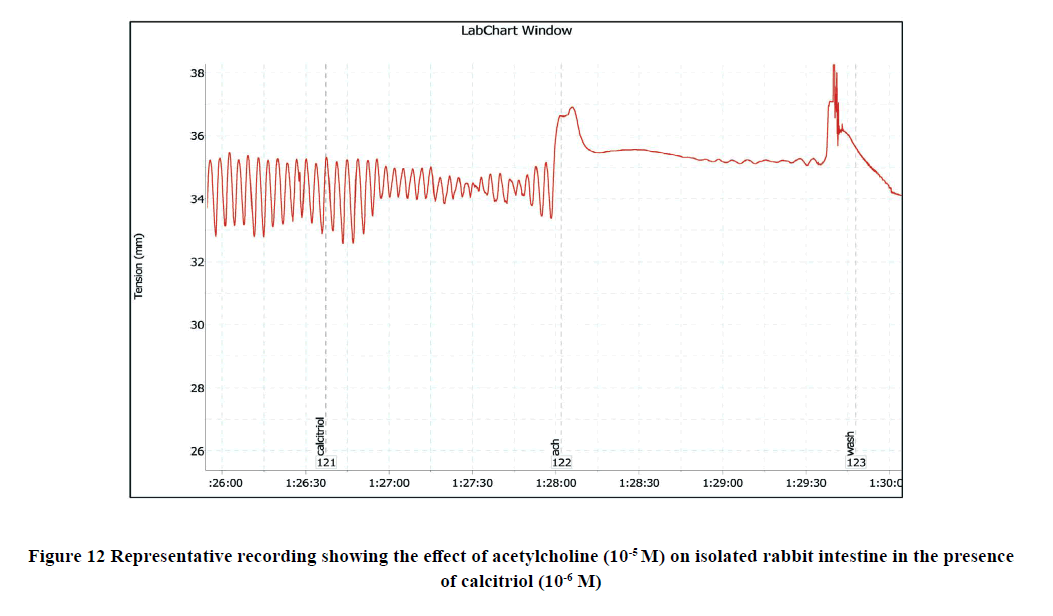
The addition of Ach (10-5 M) to the rabbit isolated ileum in the bath significantly increased the amplitude of spontaneous intestinal contractions to 3.25 ± 0.3 and decreased the frequency to 11 ± 0.38 as shown in Figure 3. However, when Ach (10-5 M) was applied on the ileal preparation after incubation with calcitriol (10-6 M), the amplitude of Achevoked contractile intestinal response was reduced to 1 ± 0.1 and the frequency was reached to 6.75 ± 0.41 as shown in Figure 12, and Figure 13.
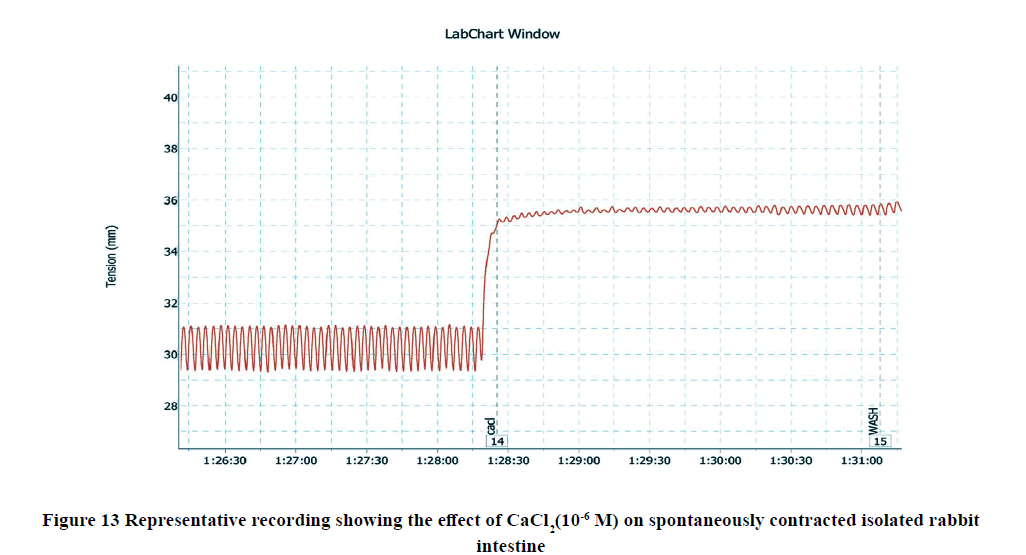
D) The Influence of Calcitriol on CaCl2 -Induced Contractions on Rabbit Isolated Ileum
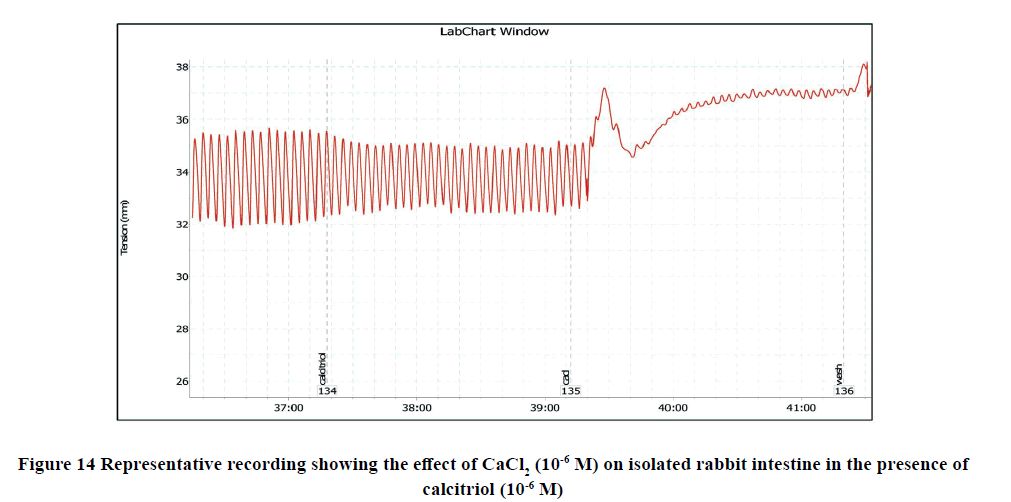
Administration of CaCl2 (10-6 M) to the bath significantly increased the amplitude of spontaneous intestinal contractions to 3.39 ± 0.3 and decreased the frequency to 10.125 ± 0.4 as shown in Figure 5. However, pretreatment with calcitriol (10-6 M) reduced the amplitude of CaCl2-induced contractions to 2.68 ± 0.4 and the frequency reached 6.875 ± 0.55 as shown in Figure 14, and Figure 15.
E) The Influence of Calcitriol on the Motility of Isolated Ileum Pre-Treated with Verapamil
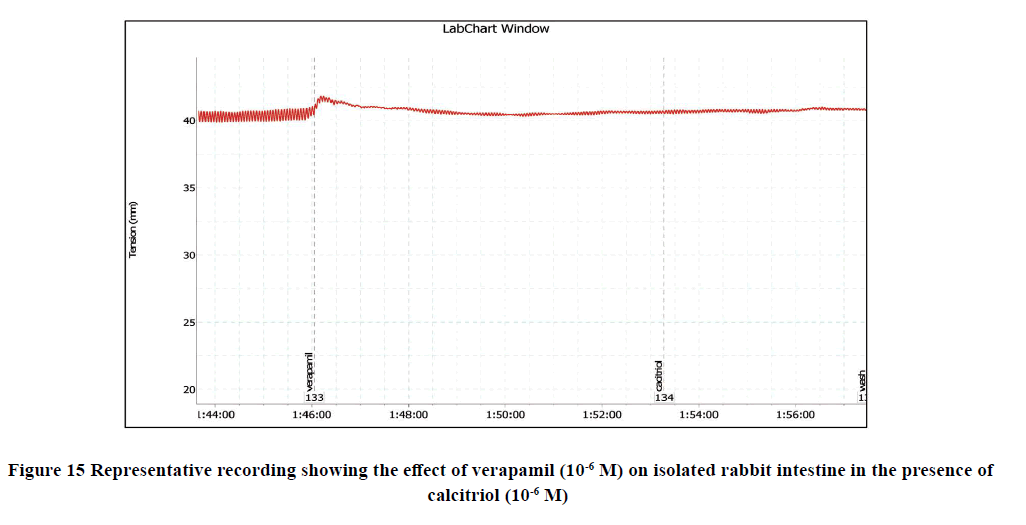
Preincubation with verapamil (10-6 M) to the bath significantly reduced the amplitude of spontaneous intestinal contractions to 1.7 ± 0 and frequency to 10.4 ± 1.2. However, pretreatment with calcitriol (10-6 M) failed to alter the relaxant response of verapamil (p>0.05) as shown in Figure 15.
Discussion
The present study was undertaken to investigate the potential effects of calcitriol on the rabbit ileal smooth muscles and explored a probable mechanism that may underlie these effects. The present study has shown that calcitriol can decline the spontaneous contractile response of the isolated rabbit ileum in a concentration-dependent manner confirming a relaxing response. These obtained results indicate that the rabbit intestinal smooth muscle is a target tissue for calcitriol that may act as a regulator of intestinal motility. The spontaneous motor activity of the ileum is a myogenic property and not nerve-mediated showing continuous and rhythmic phasic contractions [17]. The motor activity of the isolated ileum was reduced by adding calcitriol to the bath medium with a significant reduction of the contraction amplitude. Nitric oxide synthase involved in the neural Nitric Oxide (NO) synthesis is found in the myenteric nerve plexus [18]. NO production is one of the significant inhibitory neurotransmissions leading to gastrointestinal smooth muscle relaxation [19]. The mode of action of NO is to activate the K+ channels on the smooth muscle cell membrane increasing K+ ions efflux leading to cellular hyperpolarization reducing the Ca2+ ions influx through the cell membrane voltage-operated channels facilitating the smooth muscle relaxation [20-22]. Further, to explore the mechanism underlying this relaxation, the inhibitory action of calcitriol on the ileal motility was significantly reduced or even abolished by L-NAME (NO synthase inhibitor) that was added before calcitriol. Hence, this relaxant effect of calcitriol may be partly mediated by the intrinsic NO biosynthetic pathway in the rabbit ileal smooth muscle cells. These findings suggested that calcitriol might act as an intrinsic NO biosynthesis stimulator in the intestinal wall components. NO synthase could be the intermediate protein involved in the effect of calcitriol. Furthermore, the decline of the relaxation response induced by calcitriol in the presence of L-NAME may indicate that the relaxing effect of calcitriol on the ileal tissue may be possibly mediated by K+ channels activation with subsequent inhibition of voltage-operated Ca2+ channels. Previously, it was revealed that the smooth muscle contraction depends on the equilibrium between hyperpolarization by elevated K+ efflux and depolarization by declined K+ efflux as the K+ efflux regulates the Ca2+ influx [23-24]. Whether calcitriol acts as an intestinal relaxant by opening K+ channels or blocking Ca2+ channels, is a question to be answered. The excitation-contraction coupling and the subsequent contractile response in the intestinal smooth muscle in vitro were documented to be mainly mediated by the extracellular Ca2+ [25]. Both processes of phasic and tonic contractions depend on the extracellular Ca2+. Therefore, external removal Ca2+ may elicit the relaxing response [26]. However, intracellular Ca2+ sources do not significantly contribute to the tension level reached by the ileum [27]. The current findings supporting the view that calcitriol reduces the rabbit ileal contractions are in agreement with our previous observations about the relaxant effect of vitamin D3 on the rat myometrium possibly via inhibition of L-type Ca2+ channels [28]. Besides, calcitriol declined the vascular tone of the aorta of hypertensive rats via inhibition of Ca2+ influx into the endothelial cells [29]. Collectively, the relaxant effects of vitamin D3 on the uterus and aortic rings opened a window for exploration of other physiological effects of activated vitamin D3 on various tissues. It is noteworthy that pretreatment with calcitriol significantly reduced the Ach-induced contractile response in rabbit ileal smooth muscles. The contractile effect of Ach was decreased by prior administration of calcitriol compared to the maximal effect of Ach in absence of calcitriol. The binding of Ach to its muscarinic receptors (M3) in the intestinal smooth muscles can trigger a G protein with inositol 1, 4, 5-triphosphate (IP3) production resulting in the opening of calcium channels in the endoplasmic reticulum increasing calcium levels in the cytoplasm [30]. Therefore, calcitriol might have cholinergic modulation. The blocking of L-type calcium channels in the isolated rabbit ileum was studied. The relaxant effect of verapamil (L-type calcium channel blocker) on intestinal motility was recorded. The spontaneous ileal contraction was reduced but not eliminated by verapamil. The response of the isolated rabbit ileum strip to calcitriol with pre-treatment with verapamil was not evident. Calcitriol failed to modify the relaxant response of verapamil indicating that voltage-gated L-type calcium channels may be partly involved in the calcitriol-induced relaxation on the ileal tissues. Zhou, et al. showed that extracellular Ca2+ is the basis for the contractile response in vitro [25]. However, intracellular Ca2+ has a minor role in facilitating contraction in the rat distal colon smooth muscle in vitro. All these mechanisms could be applied to our results to emphasize the role of calcitriol in the rabbit ileal contractile response. Moreover, we found that the addition of CaCl2 to the isolated rabbit ileum showed evident contraction. CaCl2-induced contractions were partially antagonized by calcitriol indicating a possible inhibition of Ca2+ influx through voltage-gated calcium channels. In contrary to our results, Giraldi, et al. found that administration of calcium to the isolated guinea pig ileum, not pretreated with calcitriol, showed no contractile response [12]. Moreover, they found that calcitriol had no evident effect on the isolated ileum motility. However, when the isolated ileum strip was pre-treated by calcitriol and followed after at least 3 hours by calcium, the contractile response to calcium was apparent and continuous for 60 minutes. They concluded that vitamin D had an indirect role on isolated guinea pig ileum contractility only through facilitating the contractile response to calcium. They suggested that calcitriol may increase the calcium channel synthesis in the smooth muscle cell. We think that the discrepancies in our findings and others might be due to the variations in the drug doses and animal models. Finally, further cellular and molecular studies are required to illuminate the roles of intestinal receptors of calcitriol and involved Ca2+ and K+ channels.
Conclusion
The main strength of this study is to show that calcitriol has a relaxing activity on isolated rabbit ileum in a concentration-dependent manner with the involvement of nitric oxide pathway and calcium channels. It may play a physiological role in intestinal motility regulation. Going a step further, it could be speculated that the decline of small intestinal motility induced by calcitriol may lead to an increase in the intestinal transit time extending the contact time of nutrients in the GIT, and consequently give the chance for proper absorption of nutrients and calcium to fulfill the body needs. Further electrophysiological and molecular studies are needed.
Declarations
Acknowledgment
The authors are thankful to Zagazig Scientific and Medical Research Center (ZSMRC) members in Zagazig University, Egypt for their skillful technical assistance support.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics Approval and Consent to Participate
All experiments on rats were performed following the Animal Research Ethical Committee and performed at Zagazig Scientific and Medical Research Center (ZSMRC), Faculty of Medicine-Zagazig University, Egypt in compliance with the National Institutes of Health Guide for the care and use of laboratory animals.
References
- Bikle, Daniel D. "Vitamin D metabolism, mechanism of action, and clinical applications." Chemistry and Biology, Vol. 21, No. 3, 2014, pp. 319-29.
- Gil, Angel, Julio Plaza-Diaz, and María Dolores Mesa. "Vitamin D: Classic and novel actions." Annals of Nutrition and Metabolism, Vol. 72, No. 2, 2018, pp. 87-95.
- Carlberg, Carsten, Sabine Seuter, and Sami Heikkinen. "The first genome-wide view of vitamin D receptor locations and their mechanistic implications." Anticancer Research, Vol. 32, No. 1, 2012, pp. 271-82.
- Silva, Mariana Costa, and Tania Weber Furlanetto. "Intestinal absorption of vitamin D: A systematic review." Nutrition Reviews, Vol. 76, No. 1, 2018, pp. 60-76.
- Perwad, Farzana, and Anthony A. Portale. "Vitamin D metabolism in the kidney: Regulation by phosphorus and fibroblast growth factor 23." Molecular and Cellular Endocrinology, Vol. 347, No. 1-2, 2011, pp. 17-24.
- Wongdee, Kannikar, and Narattaphol Charoenphandhu. "Vitamin D-enhanced duodenal calcium transport." Vitamins and Hormones, Vol. 98, 2015, pp. 407-40.
- Feldman, David, Peter J. Malloy, and Coleman Gross. "Vitamin D: Biology, action, and clinical implications." Osteoporosis, Academic Press, 2001, pp. 257-303.
- Bouillon, Roger, et al. "Vitamin D and human health: Lessons from vitamin D receptor null mice." Endocrine Reviews, Vol. 29, No. 6, 2008, pp. 726-76.
- DeLuca, Hector F. "Evolution of our understanding of vitamin D." Nutrition Reviews, Vol. 66, No. suppl_2, 2008, pp. S73-S87.
- Wang, Yongji, Jinge Zhu, and Hector F. DeLuca. "Where is the vitamin D receptor?" Archives of Biochemistry and Biophysics, Vol. 523, No. 1, 2012, pp. 123-33.
- Ogut, Ozgur, and Frank V. Brozovich. "Regulation of force in vascular smooth muscle." Journal of Molecular and Cellular Cardiology, Vol. 35, No. 4, 2003, pp. 347-55.
- Giraldi, Guglielmo, et al. "Investigation of the effects of vitamin D and calcium on intestinal motility: In vitro tests and implications for clinical treatment." Acta Pharmaceutica, Vol. 65, No. 3, 2015, pp. 343-49.
- Jabeen, Q., et al. "The spasmogenic and spasmolytic activities of Lavandula stoechas are mediated through muscarinic receptor stimulation and calcium channel blockade." International Journal of Pharmacology, Vol. 3, No. 1, 2007, pp. 61-67.
- Noor, A., M. H. Najmi, and S. Bukhtiar. "Effect of montelukast on bradykinin-induced contraction of isolated tracheal smooth muscle of guinea pig." Indian Journal of Pharmacology, Vol. 43, No. 4, 2011, pp. 445-49.
- Tuladhar, B. R., Brenda Costall, and R. J. Naylor. "Modulation of 5‐HT4 receptor function in the rat isolated ileum by fluoxetine: The involvement of endogenous 5‐hydroxytryptamine." British Journal of Pharmacology, Vol. 136, No. 1, 2002, pp. 150-56.
- Tanko, Y., et al. "The effect of methanol leaves extract of Ficus glumosa on gastrointestinal motility and on castor oil induced diarrhea in laboratory animals." Journal of Natural Product and Plant Resources, Vol. 2, No. 3, 2012, pp. 360-67.
- Bani, Daniele, et al. "Relaxin depresses small bowel motility through a nitric oxide-mediated mechanism. Studies in mice." Biology of Reproduction, Vol. 66, No. 3, 2002, pp. 778-84.
- Bredt, David S., Paul M. Hwang, and Solomon H. Snyder. "Localization of nitric oxide synthase indicating a neural role for nitric oxide." Nature, Vol. 347, No. 6295, 1990, pp. 768-70.
- Jalali-Nezhad, Ahmad Ali, et al. "The effect of ginger hydroalcholic extract on rat ileal contraction in vitro." Zahedan Journal of Research in Medical Sciences, Vol. 18, No. 2, 2016, pp. 1-5.
- Almendra, Rafael B., et al. "Involvement of potassium channels, nitric oxide synthase, and guanylate cyclase in the spasmolytic effect of Simaba Ferruginea A. St.-hil on rat isolated ileum." Digestive Diseases and Sciences, Vol. 64, No. 11, 2019, pp. 3104-14.
- Baranowska, Marta, et al. "Potassium channels in blood vessels: Their role in health and disease." Advances in Hygiene and Experimental Medicine, Vol. 61, 2007, pp. 596-605.
- Nelson, Mark T., and John M. Quayle. "Physiological roles and properties of potassium channels in arterial smooth muscle." American Journal of Physiology-Cell Physiology, Vol. 268, No. 4, 1995, pp. C799-C822.
- Alexander, Stephen PH, Alistair Mathie, and John A. Peters. "Guide to Receptors and Channels (GRAC)." British Journal of Pharmacology, Vol. 153, No. S2, 2008, p. S1.
- Thorneloe, Kevin S., and Mark T. Nelson. "Ion channels in smooth muscle: Regulators of intracellular calcium and contractility." Canadian Journal of Physiology and Pharmacology, Vol. 83, No. 3, 2005, pp. 215-42.
- Zhou, Hua, et al. "Sources of calcium in agonist-induced contraction of rat distal colon smooth muscle in vitro." World Journal of Gastroenterology: WJG, Vol. 14, No. 7, 2008, p. 1077.
- Honda, Kenji, Yukio Takano, and Hiro-O. Kamiya. "Involvement of protein kinase C in muscarinic agonist-induced contractions of guinea pig ileal longitudinal muscle." General Pharmacology, Vol. 27, No. 6, 1996, pp. 957-61.
- Nouailhetas, Viviane LA, et al. "Calcium and sodium dependence of the biphasic response of the guinea-pig ileum to agonists." European Journal of Pharmacology, Vol. 116, No. 1-2, 1985, pp. 41-47.
- Hany, A., and Eman R. Abozaid. "The effect of vitamin D3 on the contractile response of isolated rat uterus." The Medical Journal of Cairo University, Vol. 87, No. December, 2019, pp. 4379-86.
- Wong, Michael SK, et al. "Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat." American Journal of Physiology-Heart and Circulatory Physiology, Vol. 295, No. 1, 2008, pp. H289-96.
- Eglen, Richard M. "Muscarinic receptor subtypes and smooth muscle function." Pharmacological Reviews, Vol. 48, 1996, pp. 531-65.