Research - International Journal of Medical Research & Health Sciences ( 2021) Volume 10, Issue 7
Drug-Eluting Stents versus Coated Stents in Diabetic Patients with Acute Coronary Syndrome
Ahmed Alsherif, Mohamed Ashraf, Ayman Moharram, Mahmoud Khaled* and Ahmed RostomMahmoud Khaled, Critical Care Medicine Department, Cairo University, Egypt, Email: noricu76@yahoo.com
Received: 02-Jun-2021 Accepted Date: Jul 23, 2021 ; Published: 30-Jul-2021
Abstract
Background: The term Acute Coronary Syndrome (ACS) is a term that describes the acute phase of ischemic coronary artery disease whether myocardial cell necrosis happens or not. Objective: To compare coated stents and drug-eluting stents in terms of Major Adverse Cardiac Events (MACE) and Instent Restenosis (ISR) rate. Patients and methods: A prospective, comparative, controlled, single-center study was conducted on forty diabetic patients diagnosed as NSTE-ACS with TIMI risk score ≥ 3, whose coronary angiography showed de novo CAD with ≥ 70% luminal stenosis to assess the influence of coated stent on the incidence of MACE and ISR after PCI in comparison with Drug-Eluting Stents (DES) in high-risk patients with ACS. Patients were divided into 2 groups according to the type of the stent; coated stent (titan 2) for group (A) and DES (Xience) for the group (B). MACE was reported during the hospital stay and after 6 months. Results: The incidence of in-hospital MACE showed no statistical significant difference between both groups, however, there was a statistically significant difference between both groups as regards the incidence of follow up MACE at 6 months mainly due to significantly higher incidence of the need for Target Lesion Revascularization (TLR) in the coated stents group. Conclusion: DES is superior to coated stents in patients with NSTE-ACS.
Keywords
ACS, PCI, DES, Coated stent, MACE
Introduction
Acute Coronary Syndrome (ACS) is a spectrum of clinical conditions characterized by acute chest pain or myocardial ischemia [1]. The spectrum of ACS encounters two categories of patients according to 12-lead Electrocardiography (ECG) and routine laboratory cardiac profile that includes ST-Segment Elevation ACS (STE-ACS) and Non-STSegment Elevation ACS (NSTE-ACS) [2]. When the TIMI risk scoring system was used, patients could be stratified across a 10-fold gradient of risk ranging from 4.7% to 40.9% (p-value<0.001) [3]. Thus, the TIMI risk score enables the identification of or allows detection of high-risk patients, who have been shown to benefit more from potent therapies such as GP IIb/IIIa inhibitors and an early invasive strategy [4]. Percutaneous Coronary Intervention (PCI) is a non-surgical invasive catheter-based procedure used to treat the narrowed coronary arteries that give blood supply to the heart muscle (myocardium). Coated stents are stents that substitute a biologically active coating to the pharmacological substance found in DES. It is commercially known as Titan 2. It is titanium-nitride-oxide coated with unique biological properties based on the stent helicoidal design with four important mechanisms of action including reduction of inflammation, inhibition of platelet aggregation and fibrin deposition, minimizing thrombogenicity by inhibiting electron transfer from fibrinogen to the coating surface, and promoting endothelial cells growth, thereby increasing the healing rates [5]. These stents are bio-compatible stents that do not release nickel-chromium and molybdenum ions; its outer layer made of protective nitride-oxide shows bioactive properties induce relaxation of smooth muscle cells is a vasodilator and reduces neointimal proliferation. Clinical trials with the coated stents have shown that they are both safe and effective for implantation. However, one complication of this approach is the reliance on the capture of circulating endothelial progenitor cells to promote re-endothelialization [5].
Patients and Methods
Our study included forty diabetic patients who were admitted to the Coronary Care Unit (CCU) of the critical care medicine department of Kasr Alainy hospital at Cairo University diagnosed as Non-ST Segment Elevation Acute Coronary Syndrome (NSTE-ACS) including Unstable Angina (UA) and Non-ST-Segment Elevation Myocardial Infarction (NSTEMI), during the period from April 2014 to January 2016; with permission from the institutional ethical committee. It was a prospective comparative single-center study.
Patients included in the study were greater than 35 years and younger than 80 years, with moderate to high-risk TIMI score (≥ 3), who was subjected to an invasive strategy, and whose coronary angiography showed single-vessel de novo Coronary Artery Disease (CAD) with a stenotic lesion causing ≥ 70% luminal reduction. Informed consent was signed by the patient or a first-degree relative. Patients fulfilling these criteria were prospectively followed up for Major Adverse Cardiac Events (MACE) including the in-hospital MACE during CCU stay and follow-up MACE for six months. Eventually, patients were classified randomly in an alternative pattern into the group (A) who were treated with coated stent (Titan 2) and group (B) who were treated with DES (Xience), each group included twenty patients.
Patients excluded in our study were younger than 35 years or older than 80 years, non-diabetic patients with low TIMI risk score (<3), those who were subjected to conservative strategy or diagnosed as ST-segment elevation acute coronary syndrome, i.e. ST-Segment Elevation Myocardial Infarction (STEMI). We excluded also patients whose coronary angiography showed a stenotic lesion causing <70% luminal reduction, in-stent restenotic lesion, twovessel or multi-vessel CAD, left main disease, ostial or bifurcation lesions, or status post-CABG and CTO lesions, patients with marked impairment of LV systolic function (LVEF<30%), renal impairment (serum creatinine >2 mg/ dl), contraindication to antiplatelet or heparin therapy (e.g. active peptic ulcer, significant external or internal bleeding, severe thrombocytopenia with platelet count <50,000/cmm and advanced liver cirrhosis), post-cardiopulmonary resuscitation status with depressed mental status, imminent co-morbid illness i.e. disseminated malignancies and chronic hematological disorders (e.g. leukemia, lymphoma, myeloproliferative diseases) were not included.
Assessment of the patients using TIMI risk score for NSTE-ACS; (1 point for each): a) age greater than or equal 65 years, b) chest pain despite the use of Aspirin in the last week, c) at least two angina episodes within the last 24 hours, d) ST-segment changes of at least 0.5 mm on admission ECG, e) elevated serum cardiac biomarkers, f) known case of CAD (coronary stenosis ≥ 50%), g) at least three risk factors for CAD, such as a current smoker, hypercholesterolemia, systemic hypertension, diabetes mellitus and positive family history of CAD [3]. TIMI Risk scoring system was applied to estimate their predicted morbidity and mortality risks as shown in Table 1 [6].
| Total score | Risk stratification | % MACE in 14 days |
|---|---|---|
| 0-2 | Low-risk patients | 4.7%-8.3% |
| 3-4 | Moderate-risk patients | 13.2%-19.9% |
| 5-7 | High-risk patients | 26.2%-40.9% |
Study Design
Patients were subjected to detailed history taking, careful physical examination, and laboratory investigations; including cardiac enzymes and renal function tests. Twelve lead ECGs and echocardiography were done to all included patients.
Angiographic data, PCI data, and Quantitative Coronary Angiography (QCA) parameters were recorded. Major Adverse Cardiac Events (MACE); including death, myocardial infarction, arrhythmia, heart failure, Target Lesion Revascularization (TLR), and Target Vessel Revascularization (TVR), were reported during the hospital stay and after 6 months. Patients were subjected to follow-up diagnostic coronary angiography after six months of PCI (whenever possible as indicated) to assess the patency of the stent and determine the need for TLR or TVR.
Quantitative Coronary Angiography (QCA)
Using edge detection method, a method of segmental artery analysis, to maximize the information obtained from coronary arteriogram. Coronary lesions were traced from two projected, perpendicular views. Magnification and distortion of the image were compensated for to determine the actual vessel profiles, using the catheter and its location as a scaling device. The two views were matched; a spatial representation of the vessel centerline is constructed mathematically, and orthogonal vessel diameters were computed at increments along this centerline. Assuming an elliptical lumen, the absolute and percentage reduction in diameter and cross-sectional area in the stenosis were computed [7].
Procedural QCA parameters include Minimal Luminal Diameter (MLD), Reference Vessel Diameter (RVD), Diameter Stenosis percent (DS%), Lesion Length (LL), and acute gain. While follow up QCA parameters include Binary Restenosis (BR), net gain, Luminal Late loss (LL), and Late Loss Index (LLI) as described in Table 2, and Table 3.
| QCA parameters | Definitions and characteristics |
|---|---|
| Minimal Luminal Diameter (MLD) | The smallest lumen diameter in the segment of interest. It ranges from 0 mm-6.0 mm. |
| Reference Vessel Diameter (RVD) | The average diameter of the coronary artery is assumed without the atherosclerotic disease. It ranges from 1.5 mm-6.0 mm. |
| Lesion length | Length of the stenosis as measured by 2 points between the angiographically normal and the diseased sub-segment. It ranges from 0 mm-60.0 mm. |
| Diameter Stenosis percent (DS%) | (RVD-MLD)/RVD. It ranges from 0%-100%. |
| Acute gain | Post-procedural MLD-Pre-procedural MLD. It ranges from 0 mm-4.0 mm. |
| QCA parameters | Definitions and characteristics |
|---|---|
| Binary Restenosis (BR) | Diameter stenosis >50% at follow-up coronary angiography in the treated coronary segment. |
| Late Luminal loss (LL) | Post-procedural MLD-MLD follow-up. It ranges from 0.1 mm-3.0 mm. |
| Late Loss Index (LLI) | Late loss/acute gain. It is a measure of the propensity for repeat vascularization. |
| Net gain | Acute gain-Late loss |
Prior to Angioplasty
All patients received; aspirin, nitroglycerin, oxygen supplementation, and 5000-10000 units of Heparin. All patients received Clopidogrel (loaded with 300 mg-600 mg at the operator’s discretion, followed by 75 mg per day). Glycoprotein IIb/IIIa receptor antagonist infusion was started before PCI and continued for 24 hours-48 hours after the procedure in some cases as indicated. Other drugs like B-blockers, anti-arrhythmic agents, inotropic and vasopressor drugs were given whenever indicated.
During the Procedure
The procedures were performed using the Siemens catheterization laboratory unit. Under clinical, electrocardiographic, and hemodynamic monitoring, coronaries were accessed mostly through the femoral artery, using the modified Seldinger technique under complete aseptic conditions. Guiding catheters and guide wires were used. Balloon and stent size selection was primarily based on visual assessment of vessel size and lesion length. Lesions were treated according to contemporary interventional techniques. The investigators used similar materials and techniques (as possible) throughout the study to maintain consistency and standardization of care. Pre-dilatation was left to the operator’s discretion. The operator decided the appropriate diameter to be implanted, aiming at a stent: vessel ratio of 1.1:1. Stents were expanded by adjusting the balloon inflation pressure to achieve an angiographic appearance of the expanded stent slightly larger than the reference vessel segment.
After Stent Deployment
Post-dilatation was allowed as necessary (at the operator’s discretion) to ensure that residual stenosis was <20% (by visual estimation) with TIMI grade 3 distal flow.
Repeat revascularization (TLR or TVR) is considered indicated if coronary angiography at follow-up shows a percent diameter stenosis ≥ 50% of previously successfully implanted stent by QCA assessment especially if there was a subjective history of recurrent angina pectoris or objective signs of ischemia at rest (ECG changes) or during exercise, presumably related to the target vessel as illustrated in Table 4.
All patients were followed up for the occurrence of Major Adverse Cardiac Events (MACE) including the in-hospital MACE during CCU stay and follow-up MACE for six months.
| Repeated re-vascularization | Description |
|---|---|
| Target Lesion Revascularization (TLR) | Any repeated revascularization of the target lesion by percutaneous intervention or bypass surgery of the target vessel is performed for restenosis or other complication of the target lesion. The target lesion is defined as the treated segment from 5 mm proximal to the stent and 5 mm distal to the stent. |
| Target Vessel Revascularization (TVR) | Any repeated revascularization by percutaneous intervention or surgical bypass of any segment of the target vessel. The target vessel is defined as the entire major coronary vessel proximal and distal to the target lesion, which includes upstream and downstream branches and the target lesion itself. |
Statistical Methods
All collected questionnaires were revised for completeness and consistency. Pre-coded data was entered on the computer system using Microsoft office Excel software “2010” program for windows seven. Obtained data were analyzed statistically by SPSS “Statistical Package for Social Science” software program, version 21. Data were summarized using ranges, mean, Standard Deviation (SD), and percentiles for quantitative numerical variables or frequency and percentages for qualitative categorical values. Statistical significance for quantitative variables was analyzed and comparison between groups was done using independent sample t-test and one-way Analysis of Variance (ANOVA) for parametric data while Mann Whitney test and Kruskal Wallis test were used for non-parametric data. The Chi-square test and Fischer exact test were used to test the statistical significance for the qualitative data.
Discriminant analysis was conducted through receiver operating characteristics analysis. Stepwise logistic regression was performed to get the significant predictors of ICU mortality. Kaplan Meier survival analysis was used to illustrate and compare cumulative mortality rates. The relationship between the studied parameters was assayed by correlations. Pearson correlation coefficient was applied to get the association between different quantitative variables. The cut-off points were used as (<0.3) for weak correlation, (0.3-0.7) for moderate correlation, and (>0.7) for strong correlation. The prevalence rate was determined from the number of identified cases at the time of the study divided by all patients examined. p-values ≤ 0.05 were considered statistically significant. Illustrative graphs were used to demonstrate obtained data.
Results
Forty patients including 19 males and 21 females with an age group ranging from 50 to 72 years old fulfilled the criteria and were enrolled in the study.
Demographic Data
There was no significant difference between both groups regarding gender distribution (p-value 0.500), mean age (p-value 0.691), risk factors of CAD (p-value 0.39), TIMI risk score (p-value 0.1), associated co-morbidities (p-value 0.07), coronary vessel affected (p-value 0.620) or segment affected (p-value 0.862).
Procedural Data
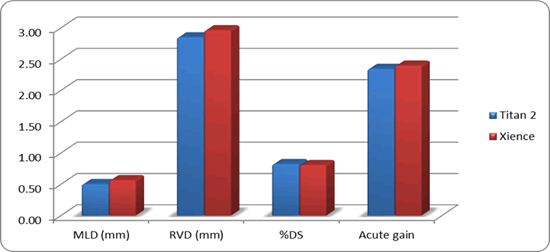
QCA was done during coronary angiography before and after PCI and revealed that there were no significant differences between both groups as regard pre-procedural MLD, RVD, DS%, and acute gain. However, there was a statistically significant difference between both groups regarding lesion length. Patients of group B had longer lesions compared to those of group A, (29.2 ± 7.2 for group ‘’B’’ vs. 21.4 ± 7.3 for group ‘’A’’, p-value 0.002) as explained by Table 5 and Figure 1.
| Procedural QCA | Group A | Group B | p-value |
|---|---|---|---|
| Lesion length (mm) | 21.4 ± 7.3 | 29.2 ± 7.2 | 0.002 |
| MLD | 0.50 ± 0.28 | 0.57 ± 0.20 | 0.425 |
| RVD | 2.85 ± 0.33 | 2.96 ± 0.28 | 0.254 |
| DS% | 0.83 ± 0.10 | 0.81 ± 0.07 | 0.515 |
| Acute gain | 2.35 ± 0.40 | 2.40 ± 0.33 | 0.666 |
There was a statistically significant difference between both groups as regard stent length. Patients of the group (B) underwent PCI using (Xience) DES with longer stents (mean stent length of 30.7 ± 7.3 mm) compared to patients of the group (A) who underwent PCI using (Titan 2) BAS with shorter stents (mean stent length of 23.4 ± 6.9 mm); (p-value 0.002). There was a statistically significant difference between both groups as regards the pre-dilatation technique (50% for group A versus 80% for group B, p-value 0.048). There was no statistically significant difference between both groups regarding procedural complications (p-value 0.244).
Follow up Data
There was no statistically significant difference between both groups as regard in-hospital MACE (p-value 0.115). However, there was a statistically significant difference between both groups as regard incidence of cumulative follow-up MACE after 6 months (p-value 0.046), specifically the need for TLR (p-value 0.024) as demonstrated in Table 6, and Table 7.
| MACE | Group (A) | Group (B) | p-value | ||
|---|---|---|---|---|---|
| In-hospital MACE | 3 | (15%) | 0 | (0%) | 0.115 |
| Follow-up (mid-term) MACE | 6 | (30%) | 1 | (5%) | 0.046 |
| Follow up MACE at 6 months | Group (A) | Group (B) | p-value | ||
|---|---|---|---|---|---|
| Unstable Angina | 3 | (15%) | 1 | (5%) | 0.302 |
| Non-fatal Myocardial Infarction | 2 | (10%) | 0 | (0%) | 0.244 |
| TLR | 5 | (25%) | 0 | (0%) | 0.024 |
| Mortality (Fatal MI) | 1 | (5%) | 0 | (0%) | 0.5 |
Seven patients of our study (17.5%) reported MACE at 6 months as follow: four patients developed recurrent anginal pains; of whom three patients belonged to group (A) and needed TLR, and one patient belonged to the group (B) and was managed conservatively with no need for TLR. Two patients who belonged to group (A) had a myocardial infarction and needed TLR; One of them developed acute cardiogenic shock and died during the ICU stay as seen in Table 7.
Six patients from the group (A) underwent follow-up coronary angiography after six months of PCI versus five patients from the group (B). There was a higher incidence of significant binary restenosis (>50% luminal reduction) after 6 months in patients of group A (10%) compared to group (B) (5%), but this was of no statistical significance; p-value 0.053.
QCA analysis was repeated after 6 months during the period of follow up for both groups as indicated and revealed that there were statistically significant differences between both groups as regard late loss (0.5 ± 1.0 for group “A” versus 0.0 ± 0.1 for group “B”, p-value 0.049) and late loss index (23 ± 48% for group “A” versus 0.1 ± 5% for group “B”, p-value 0.05) as described in Table 8.
| Follow up QCA | Group (A) | Group (B) | p-value |
|---|---|---|---|
| Late loss | 0.5 ± 1.0 | 0.0 ± 0.1 | 0.049 |
| Net gain | 1.9 ± 1.0 | 2.4 ± 0.4 | 0.069 |
| Late loss index | 23 ± 48% | 0.1 ± 5% | 0.05 |
Discussion
Many studies demonstrated the impact of DES versus coated and/or metallic stents on the outcome of PCI [10- 13]. Our study observed that the use of coated stent in PCI was associated with unfavorable outcomes as regard its statistically significant higher frequencies of MACE and the need for TLR within 6 months compared to DES. Thus, our work concluded that the use of DES was superior to the coated stent in patients with NSTE-ACS candidates for invasive strategy (PCI).
Our results were supported by Moses, et al. that demonstrated a decrease in the frequency of the need for revascularization of the target lesion with DES (16.6% in metallic stent group versus 4.1% in the DES group, p-value<0.001) [14]. In agreement with our findings, Stone, et al. observed that target lesion revascularization was required in 3% of the group that received DES, as compared with 11.3% of the group that received a metallic stent (p-value<0.001) [15].
In contrast to our results, Base-ACS Trial described that the incidence of Myocardial Infarction (MI) at 24 months was statistically significantly greater with the Xience group compared to Titan 2 group (6.8% versus 2.9%; p-value 0.009) [13]. However, the BASE-ACS trial concluded that the incidence of cumulative MACE at 24 months was relatively lower with Titan 2 group than with the Xience group (11.3% versus 12.4%) with no statistical insignificance (p-value 0.37). In 2016, a final report of the Base-ACS trial with a long-term follow-up period of 5 years was produced in patients with ACS, bio-active stent was also non-inferior to an everolimus-eluting stent for MACE (14.4% versus 17.8%, respectively; p-value<0.001). The rates of cardiac death and ischemia-driven TLR were comparable (2.8% versus 3.8%, and 8.3% versus 9.9%; p-value 0.76 and 0.58, respectively) [16].
Our study did not agree with the TiNOX trial stated that there was a significant reduction in MACE in titan stent at six months and five years (7% versus 27%; p-value 0.02, and 16% versus 39%; p-value 0.03 respectively), largely driven by a significantly reduced need for tar-get-lesion revascularization (9% versus 25%) [17]. However, this trial compared the outcome of Titan 2 stent with bare-metal stent only without comparing it with DES.
Unlikely, the PORI registry reported that Titan 2 stent achieved an excellent clinical outcome over 5-year follow-up, with a significantly lower incidence of MI (9.5% versus 20.6%, p-value 0.002) and cumulative MACE at 5 years (16.9% versus 26%, p-value 0.03) as compared to DES [18]. However, only 17.5% of registry patients were diabetic and the study included only 244 ACS patients out of 405. Besides, this registry compared titan 2 stents with a different type of stent (Paclitaxel eluting) other than that used in our study (Everolimus eluting).
Limitations of the Study
The number of the study population might be relatively low especially for the detection of clinical events in addition to the relatively short follow up duration.
References
- Bassand, Jean-Pierre, et al. "Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: The task force for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes of the European Society of Cardiology." European Heart Journal, Vol. 28, No. 13, 2007, pp. 1598-1660.
- Authors/Task Force Members, et al. "ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The task force for the management of Acute Coronary Syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC)." European Heart Journal, Vol. 32, No. 23, 2011, pp. 2999-3054.
- Antman, Elliott M., et al. "The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making." JAMA, Vol. 284, No. 7, 2000, pp. 835-42.
- Cannon, Christopher P. "Oral platelet glycoprotein IIb/IIIa receptor inhibitors-part I." Clinical Cardiology: An International Indexed and Peer‐Reviewed Journal for Advances in the Treatment of Cardiovascular Disease, Vol. 26, No. 8, 2003, pp. 358-64.
- Karjalainen, Pasi P., et al. "Titanium-nitride-oxide-coated Titan-2 bioactive coronary stent: A new horizon for coronary intervention." Expert Review of Medical Devices, Vol. 7, No. 5, 2010, pp. 599-604.
- Brown, C., B. McNicholl, and R. Wright. "‘‘America’’: A mnemonic for the TIMI risk score." Emergency Medical Journal, Vol. 25, No. 2, 2008, p. 122.
- Brown, B. GREG, et al. "Quantitative coronary arteriography: Estimation of dimensions, hemodynamic resistance, and atheroma mass of coronary artery lesions using the arteriogram and digital computation." Circulation, Vol. 55, No. 2, 1977, pp. 329-37.
- Garrone, Paolo, et al. "Quantitative coronary angiography in the current era: Principles and applications." Journal of Interventional Cardiology, Vol. 22, No. 6, 2009, pp. 527-36.
- Fiorella, David J., et al. "Target lesion revascularization after wingspan: Assessment of safety and durability." Stroke, Vol. 40, No. 1, 2009, pp. 106-10.
- Angioi, Michaël, et al. "French Ministry of Health prospective multicentre study using bio-active stents coated with titanium nitride oxide: The EVIDENCE registry." Archives of Cardiovascular Diseases, Vol. 105, No. 2, 2012, pp. 60-67.
- Chavarri, Mariano Valdés, et al. "Titanium-nitride-oxIde-coated stents multicenter registry in diabetic patienis: The TIBET registry." Heart and Vessels, Vol. 27, No. 2, 2012, pp. 151-58.
- Karjalainen, Pasi P., and Wail Nammas. "Titanium-nitride-oxide-coated coronary stents: Insights from the available evidence." Annals of Medicine, Vol. 49, No. 4, 2017, pp. 299-309.
- Karjalainen, Pasi P., et al. "A prospective randomised comparison of titanium-nitride-oxide-coated bioactive stents with everolimus-eluting stents in acute coronary syndrome: The BASE-ACS trial." EuroIntervention, Vol. 8, No. 3, 2012, pp. 306-15.
- Moses, Jeffrey W., et al. "Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery." New England Journal of Medicine, Vol. 349, No. 14, 2003, pp. 1315-23.
- Stone, Gregg W., et al. "A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease." New England Journal of Medicine, Vol. 350, No. 3, 2004, pp. 221-31.
- Karjalainen, Pasi P., et al. "Long-term clinical outcome of titanium-nitride-oxide-coated stents versus everolimus-eluting stents in acute coronary syndrome: Final report of the BASE ACS trial." International Journal of Cardiology, Vol. 222, 2016, pp. 275-80.
- Moschovitis, Aris, et al. "Randomised comparison of titanium-nitride-oxide coated stents with bare metal stents: Five year follow-up of the TiNOX trial." EuroIntervention, Vol. 6, No. 1, 2010, pp. 63-68.
- Karjalainen, Pasi P., et al. "Five‐year clinical outcome of titanium‐nitride‐oxide‐coated bioactive stent implantation in a real‐world population: A comparison with paclitaxel‐eluting stents: The PORI registry." Journal of Interventional Cardiology, Vol. 24, No. 1, 2011, pp. 1-8.
Declarations
Conflicts of Interest
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Data Availability Statement
Data of the current study is available in the critical care medicine department at Cairo University and authors can make data available on request through the data access committee and local institutional board. The contact person is Dr. Ahmed Rostom, email: annrostom@hotmail.com.
Conclusion
The use of coated stents did not show a statistically significant higher incidence of in-hospital MACE compared to DES. However, a statistically significant higher incidence of follow-up MACE within six months of PCI with coated stent was demonstrated mainly because of statistically significant increased frequency of the need for Target Lesion Revascularization (TLR) in comparison with DES in moderate to high-risk diabetic patients with NSTE-ACS. So, we concluded the inferiority of coated stent over DES in PCI of critically ill ischemic patients with diabetes mellitus.