Research - International Journal of Medical Research & Health Sciences ( 2021) Volume 10, Issue 4
Effect of Non-Surgical Periodontal Therapy on Salivary pH in Periodontal Disease: A Randomized Clinical Trial
Stuti Gupta1*, Shradha2, Ejya Sharma3, Himani Sharma1, Saransh Srivastava1 and Radhika Gupta12Oxford Dental College, Bangalore, Karnataka, India
3Periodontist and Implantologist, Private Practitoner, New Delhi, India
Stuti Gupta, School of Dental Sciences, Sharda University, Greater Noida, India, Email: Stuti.gupta@sharda.ac.in
, DOI: 0
Abstract
Aim: The study aims to assess the effect of pre and post Non-Surgical Periodontal Therapy (NSPT) on salivary pH in patients suffering from chronic periodontitis. Materials and Method: Patients reporting to the Department of Periodontics, I.T.S Dental College, Hospital and Research Centre, Greater Noida, Uttar Pradesh are included in the study. It’s a randomized clinical trial where patients are randomized into two groups of generalized chronic gingivitis and generalized chronic periodontitis. The sample size was 30 patients and the period of study was 2 months. Unstimulated saliva is collected at the first visit when a patient reports to the OPD for NSPT. Clinical parameters are recorded and NSPT is performed for the patient. Post 14 days of the treatment, saliva is collected and analyzed for pH and clinical parameters are recorded again. pH is determined using a pH meter. Data is collected and statistically interpreted. Statistical testing to be used is Mann Whitney U Test, Chi-square test, and Paired t-test. Results: There was a statistically significant difference in plaque index, gingival index, and oral hygiene index between the two groups. There was a definite reduction in pH post Scaling and Root Planning (SRP) but it was statistically non-significant due to the relatively smaller sample size. Further research is required to substantiate the results for the same.
Keywords
Salivary pH, Salivary biomarkers, Periodontitis, SRP, NSPT
Introduction
Oral diseases such as dental caries, gingivitis, and periodontitis are initiated at the interface between microbial ecosystem and host tissue [1].
The diagnosis of active phases of periodontal disease and the identification of patients at risk for active disease represent a challenge for both clinical investigators and clinicians. In general, clinical parameters such as probing depth, attachment level, bleeding on probing, plaque, and gingival indices are used to assess disease severity.
Saliva is a dilute fluid, over 99% being made up of water. With a multitude of biomarkers, the salivary pH may be used as a quick chair-side test.
Saliva has a pH normal range of 6.2 to 7.6 with 6.7 being the average pH. A saliva pH below 7.0 usually indicates academia (abnormal acidity of the blood). If a chronic condition exists the mouth is more susceptible to dental decay, halitosis, and periodontitis. A saliva pH above 7.0 usually indicates alkalinity. Excessive alkalinity can bring about the same anaerobic conditions as academia [1].
The two key factors to plaque formation are first there must be oral bacteria to attack food particles and elevate the pH. Second, the pH must elevate above 7.6 to grow dental plaque that causes periodontal disease [2,3]. Therefore, such a study is necessary to establish the nature of the exact role in the induction and progression of periodontal disease.
Materials and Methods
Materials
Mouth Mirror, Dental Explorer (No.17/23), Cotton Plier, UNC-15 Face Mask, Gloves, Disclosing Agent, Eppendroff tubes, and pH meter.
Inclusion Criteria
1. Systemically healthy individuals aged between 25-54 years
2. Patients suffering from Stage 1 and Stage 2 Periodontitis (2017 Classification) were included in the study
3. Patient willing to give informed consent were included in the study
4. No usage of antibiotics in the preceding 6 months of the study
5. No history of periodontal therapy 6 months before the study
Exclusion Criteria
1. Patients with conditions like Diabetes mellitus, hypertension, atherosclerosis, and other systemic diseases known to affect periodontal status
2. Females with pregnancy and lactating mothers
3. Patients with deleterious habits like alcohol consumption, tobacco habits, and any other adverse habits
4. Patients who have received any surgical or non-surgical periodontal therapy in the past 6 months
5. Patients who have received any antimicrobial therapy in the past 3 months
6. Patients suffering from Salivary Gland Diseases
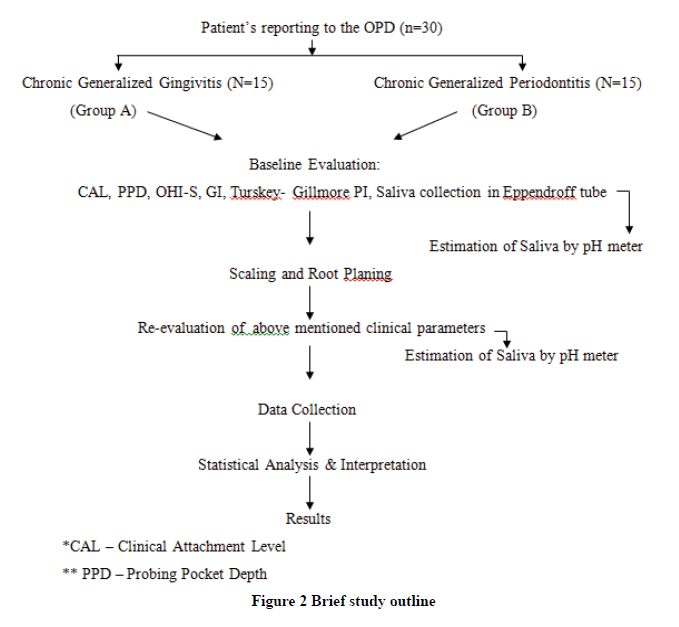
Procedure
Un-stimulated Saliva is collected at the first visit when a patient reports to the OPD for NSPT.
Clinical Parameters are recorded and NSPT is performed for the patient. Post 14 days of the treatment, saliva is collected and analyzed for pH and clinical parameters are recorded again. pH is determined using a pH meter (Figure 1). Data is collected and statistically interpreted. Clinical parameters recordings include Gingival Index, Plaque Index, and Oral Hygiene Index-Simplified (Figure 2).
Results
In this study, 50 subjects were evaluated for indices, periodontal parameters, and pH levels before and after scaling and root planing.
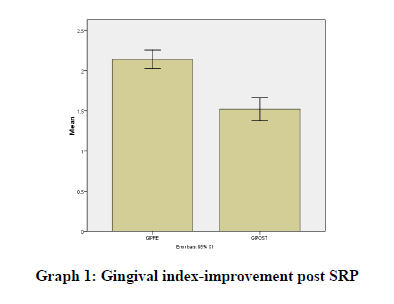
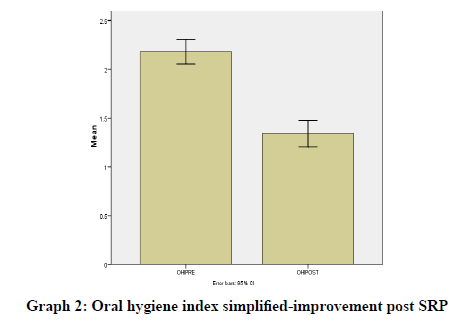
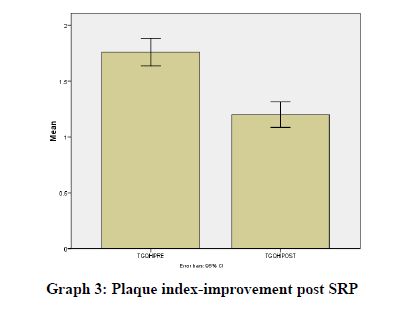
Results were statistically analyzed using a t-test and Wilcoxon test. It was seen that there was a clinically significant difference in the gingival index (Graph 1), oral hygiene indices (Graph 2), plaque indices (Graph 3), and periodontal parameters post SRP which suggests improvement in periodontal condition and reduction in inflammatory markers (Table 1).
Mean |
N |
Std. Deviation |
Std. Error Mean |
||
|---|---|---|---|---|---|
Pair 1 |
GIPRE |
2.14 |
50 |
0.405 |
0.057 |
GIPOST |
1.52 |
50 |
0.505 |
0.071 |
|
Pair 2 |
TGQHIPRE |
1.76 |
50 |
0.431 |
0.061 |
TGQHIPOST |
1.2 |
50 |
0.404 |
0.057 |
|
Pair 3 |
OHIPRE |
2.18 |
50 |
0.438 |
0.062 |
OHIPOST |
1.34 |
50 |
0.479 |
0.068 |
|
Pair 4 |
PPDPRESRP |
2.48 |
50 |
0.49 |
0.069 |
PPDPOSTSRP |
1.94 |
50 |
0.487 |
0.069 |
|
Pair 5 |
CALPRESRP |
2.63 |
50 |
0.637 |
0.090 |
CALPOSTSRP |
2.1 |
50 |
0.625 |
0.088 |
|
Pair 6 |
SALIVARYpHPRESRP |
7.9 |
50 |
0.755 |
0.107 |
SALIVARYpHPOSTSRP |
7.5 |
50 |
0.497 |
0.070 |
|
The difference in pH of saliva was also seen post SRP suggesting a reduction in microbes which cause saliva to be alkaline thus high pH, but the difference was not statistically significant which may be due to less sample size (Table 2).
Paired Differences |
t |
df |
Sig. (2-tailed) |
||||||
|---|---|---|---|---|---|---|---|---|---|
Mean |
Std. Deviation |
Std. Error Mean |
95% Confidence Interval of the Difference |
||||||
Lower |
Upper |
||||||||
Pair 1 |
GIPRE-GIPOST |
0.62 |
0.49 |
0.069 |
0.481 |
0.759 |
8.941 |
49 |
0 |
Pair 2 |
TGQHIPRE-TGQHIPOST |
0.56 |
0.501 |
0.071 |
0.417 |
0.703 |
7.897 |
49 |
0 |
Pair 3 |
OHIPRE-OHIPOST |
0.84 |
0.422 |
0.06 |
0.72 |
0.96 |
14.08 |
49 |
0 |
Pair 4 |
PPDPRESRP-PPDPOSTSRP |
0.534 |
0.331 |
0.047 |
0.44 |
0.628 |
11.404 |
49 |
0 |
Pair 5 |
CALPRESRP-CALPOSTSRP |
0.529 |
0.405 |
0.057 |
0.414 |
0.644 |
9.242 |
49 |
0 |
Pair 6 |
SALIVARYpHPRESRP-SALIVARYpHPOSTSRP |
0.392 |
0.471 |
0.067 |
0.258 |
0.526 |
5.883 |
49 |
0 |
Discussion
Gingivitis and periodontitis are the most prevalent chronic inflammatory conditions that affect as much as 80% of the adult population [4-8]. The activation of the patient’s host response liberates a myriad of metabolic byproducts at the interface between the tooth and the periodontal pocket which leads to the release of destructive cellular enzymes, cytokines, chemokines, and other mediators of tissue destruction [9]. There are numerous markers in saliva that have been proposed and used as diagnostic tests for periodontal disease but the diagnostic tests should demonstrate sensitivity and specificity. It is improbable that a single marker will prove to be both sensitive and specific due to the complexity of the periodontal disease.
A saliva pH of 7.0 usually indicates a healthy dental and periodontal situation. At this pH, there is a low incidence of dental decay combined and little or no calculus. Therefore, stable conditions should be found in this environment.
A saliva pH below 7.0 usually indicates acidemia (abnormal acidity of the blood). If a chronic condition exists, the mouth is more susceptible to dental decay, halitosis, and periodontitis. Chronic acidemia can be a causative factor for a multitude of diseases affecting the whole body.
A saliva pH above 7.0 usually indicates alkalinity. Excessive alkalinity can bring about the same anaerobic conditions as acidemia, but it is a much rarer condition.
Takahashi, et al. concluded in their study that the periodontopathogens grow at a mildly acidic pH [9]. This is following our result for the pH of chronic periodontitis.
Fujikawa, et al. studied the correlation between the pH level and the microflora in periodontal pockets in the various stages of periodontal disease [10]. A change in pH level was seen in deep pockets or severe gingival inflammation. A close correlation was seen between salivary and crevicular pH. The pH level was significantly positively related to the proportion of coccoid forms but was negatively correlated with the proportion of motile organisms that are reported to be related to periodontal disease.
Galgut conducted a study to investigate any possible correlations between pH and gingivitis and periodontal pockets [11]. Correlations between pH and gingivitis were not identified, but significant correlations between pH and periodontal pockets were evident.
Thus, this study establishes the importance of scaling and root planning in controlling periodontal disease beforehand using Non-Surgical therapy and the value of salivary pH as an important and easy chair-side tool for the diagnosis of periodontal diseases.
Conclusion
Saliva is a fluid that can be easily collected, contains locally-derived and systemically derived markers of periodontal disease, and hence may offer the basis for a patient-specific diagnostic test for periodontitis.
Saliva can be used as an indicator of prognosis during periodontal treatment. Within the limitations of this study, it has been observed that there is a correlation between the pH of saliva and periodontal diseases when compared with healthy groups. Salivary pH in patients with chronic generalized gingivitis was more alkaline than that in patients with clinically healthy gingiva. In patients with chronic generalized periodontitis, the salivary pH was more acidic than the control group. This may be of diagnostic value in the future, but further elaborate studies with larger sample size, microbiological analysis, and ions in the salivary sample are needed to draw definite conclusions.
Declarations
Conflicts of Interest
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
References
- Baliga, Sharmila, Sangeeta Muglikar, and Rahul Kale. "Salivary pH: A diagnostic biomarker." Journal of Indian Society of Periodontology, Vol. 17, No. 4, 2013, pp. 461-65.
- Bezerra Júnior, Arnaud Alves, et al. "Evaluation of organic and inorganic compounds in the saliva of patients with chronic periodontal disease." Odonto Science Magazine, Vol. 25, No. 3, 2010, pp. 234-38.
- Shaila, Mulki, G. Prakash Pai, and Pushparaj Shetty. "Salivary protein concentration, flow rate, buffer capacity and pH estimation: A comparative study among young and elderly subjects, both normal and with gingivitis and periodontitis." Journal of Indian society of Periodontology, Vol. 17, No. 1, 2013, pp. 42-46.
- Fiyaz, Mohamed, et al. "Association of salivary calcium, phosphate, pH and flow rate on oral health: A study on 90 subjects." Journal of Indian Society of Periodontology, Vol. 17, No. 4, 2013, pp. 454-60.
- Kejriwal, Swati, et al. "Estimation of levels of salivary mucin, amylase and total protein in gingivitis and chronic periodontitis patients." Journal of Clinical and Diagnostic Research: JCDR, Vol. 8, No. 10, 2014, pp. ZC56-ZC60.
- Patil, Abhilasha S., et al. "Evaluation of salivary biomarkers of periodontitis among smokers and nonsmokers: A novel study." Journal of Family Medicine and Primary Care, Vol. 9, No. 2, 2020, pp. 1136-42.
- Kim, Jeffrey J., Christine J. Kim, and Paulo M. Camargo. "Salivary biomarkers in the diagnosis of periodontal diseases." Journal of the California Dental Association, Vol. 41, No. 2, 2013, pp. 119-24.
- Giannobile, William V. "Salivary diagnostics for periodontal diseases." The Journal of the American Dental Association, Vol. 143, 2012, pp. 6S-11S.
- Takahashl, N., et al. "Acid tolerance and acid‐neutralizing activity of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum." Oral Microbiology and Immunology, Vol. 12, No. 6, 1997, pp. 323-28.
- Fujikawa, K., et al. "pH determination in human crevicular fluids. Examination of the pH meter and evaluation of the correlation between pH level and clinical findings or the microflora in each periodontal pocket." Journal of the Japanese Society of Periodontology, Vol. 31, No. 1, 1989, pp. 241-48.
- Galgut, P. N. "The relevance of pH to gingivitis and periodontitis." Journal of the International Academy of Periodontology, Vol. 3, No. 3, 2001, pp. 61-67.