Research Article - International Journal of Medical Research & Health Sciences ( 2025) Volume 14, Issue 2
Effectiveness of Some Types of Toothpastes in the Removal of Dental Plaque and Tartar
Amani Laheq1, Eid Ibrahim Brima1,2, Abdullah Dhafer Alasmari3, Taher Sahlabji1 and Mohammed Elimam Ahamed Mohammed1*2Department of Health and Life Sciences, School of Allied Health Science, de Montfort University, The Gateway, Leicester LE1 9BH, UK
3Department of Medical Forensic Chemistry, The Poison Control and Medical Forensic Chemistry Center, Asir Region, King Abdullah Road, Abha 62221, Saudi Arabia
Mohammed Elimam Ahamed Mohammed, Department of Chemistry, King Khalid University, Abha, Saudi Arabia, Email: meaahmad@kku.edu.sa
Received: 21-Oct-2023, Manuscript No. IJMRHS-23-117918; Editor assigned: 24-Oct-2023, Pre QC No. IJMRHS-23-117918 (PQ); Reviewed: 07-Nov-2023, QC No. IJMRHS-23-117918; Revised: 01-Apr-2025, Manuscript No. IJMRHS-23-117918 (R); Published: 08-Apr-2025
Abstract
Objective: This article investigated the efficiency of some toothpastes in the removal of dental plaque and tartar using nine upper and lower sheep jaws.
Method: The used toothpastes were Sensodyne, Colgate, Closeup blue, Closeup green, Closeup red, Aloe dent, Meridol, Colgate kids and Crest kids. The efficiency was examined by measuring some of the plaque and tartar constituents in toothpaste solutions (before and after brushing). The measured parameters are the pH, Total Dissolved Solids (TDS), conductivity, total carbohydrates, total proteins and some minerals (sodium, potassium, calcium and magnesium).
Results and discussion: Most of the studied parameters were significantly increased in the after brushing toothpastes solutions. Some after brushing toothpastes solutions were characterized by significantly decreased sugars, proteins and minerals. The studied toothpastes exerted variable significant effects on the studied parameters which may be due to the different chemical constituents.
Conclusion: Colgate kids’ toothpaste was the most effective in the removal of plaque and tartar followed by Sensodyne and Colgate adults. The Colgate adult is the best in sodium and calcium re-mineralization.
Keywords
Proteins, Sugars, pH, Conductivity, Anthrone, BradfordIntroduction
Toothpastes are used to improve oral hygiene through preventing and fighting dental diseases like caries. Furthermore, toothpastes help to remove food and dental plaque and tartar from the teeth [1,2].
Toothpastes are composed of major and minor components depending on their percentage or concentration. The major constituents of the toothpastes are the water (20-40%) and the abrasives (50%). The popular abrasives used in the toothpastes are calcium carbonate, calcium hydrogen phosphate, hydroxyapatite, aluminum hydroxide and silica. The minor components include:
• Fluoride (1450 ppm) for the prevention of dental caries and re mineralization of teeth. Fluoride is added to the toothpastes as sodium fluoride, sodium monofluorophosphate and stannous fluoride.
• Antibacterial agents including zinc chloride and triclosan.
• Detergents, predominantly Sodium Lauryl Sulfate (SLS).
• Re mineralization compounds such as the fluoride, hydroxyapatite and calcium phosphate.
• Humectants and binders like the glycerol Polyethylene Glycol (PEG), xylitol and sorbitol as humectants and binders such as natural gums (gum Arabic) and polysaccharides such as the carrageenan and the synthetic cellulose (carboxymethyl and hydroxethyl cellulose).
• Anti-calculus compounds including the zinc citrate and sodium polyphosphate.
• Antisensitivity molecules as the arginine, strontium chloride and potassium nitrate [3-5].
Dental plaque is a non-mineralized layer or filament contact to the surface of tooth. It is redefines as biofilm which is composed of bacterial layer (dead and alive), bacterial extracellular products and salivary glycoproteins such as agglutinins and IgA [6-8]. The presence of dental plaque induces inflammatory response in the tissues surrounding the teeth and it is associated with dental caries, periodontal diseases and gingivitis [9].
Dental tartar or calculus is an inorganic layer or a calcified dental plaque on the outer surface of teeth which is rich in calcium and phosphate. The tartar layer has a yellow or black color and a hard surface [10-12]. Dental tartar is classified as a minor factor that causes periodontal disease. Periodontal diseases are caused by infections or inflammations of the gums and mouth bones [11,13].
The aim of this study was to investigate the efficiency of different toothpastes in the removal of plaque and tartar through measuring some minerals, carbohydrates, proteins, pH and Electrical Conductivity (EC) in toothpastes solution before and after oral brushing for sheep jaws.
The contacted previous studies were associated with investigating the effect of toothpaste in the removal of plaque through measuring the plaque index or score [14,15]. To our knowledge, this is the first study to measure the constituents of the plaque in the after-brushing solution.
Materials and Methods
Study samples and their treatment
Nine types of toothpaste brands were randomly, purchased from markets in Abha and Khamis Mushait-Kingdom of Saudi Arabia. From each type of the toothpastes, three samples were bought. Sensodyne, Colgate, close up (blue, green, and red), Aloe dent and Meridol, beside Colgate and crest for kids. Twenty-seven sheep jaws (14 lower and 13 upper) were brushed by solutions of toothpastes. The solutions of toothpastes were prepared by dissolving 2.8 g of each toothpaste in 30 mL of de-ionized water (9.3%; W/V). Sodium azide was added to the toothpaste solution to prohibit microbial contamination (0.9% W/V). Moreover, small amount of Triton X-100 was added as a surfactant to facilitate the homogenization of the toothpastes solutions [16]. After that the prepared toothpastes solutions were divided to two parts; 10 ml for the measurement of the studied parameters in the toothpastes solutions before the brushing process and 20 ml for the brushing of the jaws teeth and measurement of the parameters after brushing.
Analysis of the studied parameters
The pH, EC and TDS of the toothpaste solutions were determined using the multi-parameter meter of HANNA (portable pH/EC/TDS-Meter-HI9810-6). The measurement was carried out after calibrating the meter with pH 4.0 and pH 7.0 buffers and a solution of potassium chloride with EC value of 1413 μS/cm (7.45 g/L). Ten mLs of each toothpaste solution was used for the determination of the three parameters and the measurements were conducted in duplicate to investigate the errors of the meter, pipette and hands.
The carbohydrates were measured spectrophotometrically using slightly modified anthrone method [17]. The modification was using half of the amounts mentioned in the original method as follows: 2 mL of each toothpaste solution was added to 4 mL of Anthrone reagent (2 grams of anthrone in one liter of 95% sulfuric acid). The mixture was mixed using vortex mixer and left for 10 minutes. After that the absorbance of the solution was measured using Laheq, et al. Int J Med Res Health Sci, 2025, 14 (2): 1-15 Jenway spectrophotometer (6850 UV/Vis) at the wavelength of 540 nm. On the other hand, a standard curve of glucose was created using serial dilution in the range of (0.0-100 mg/dl).
The protein concentration was determined according to the Bradford spectrophotometric method [18]. 100 μL of the toothpaste solution was reacted with 5 mL of coomassie blue reagent and the absorbance was measured at the wavelength of 595 nm. Standard curve was created using albumin in the range of (0.0- 500 μg/mL). The Coomassie blue reagent was prepared as follows: 100 mg of coomaaie brilliant blue G-250 was dissolved in 50 mL of 95% ethanol, 100 mL of 85% (W/V) phosphoric acid was added and finally, the solution was diluted to one liter using deionized water and filtered.
The sodium, potassium and calcium were measured in the toothpaste solutions using the flame photometer (Flame Photometer PFP7-JENWAY). For the creation of the standard curves, the following standards were prepared; the standards of the calcium were 0.1,0.5,1,2,4,6,8, and 10 milligrams per 100 milliliter (mg/100 ml), potassium standards were 0.1,0.5,1,2 and 4 milligrams per 100 milliliter (mg/100 ml) while the standards of sodium were 0.1, 0.5, 1, 2, 4, 6, 8, 10 and 50 mg/100 ml. The emission wavelengths of the calcium, potassium, and sodium were 622 nm (orange), 766 nm (violet), and 589 nm (yellow), respectively. Quality control samples were prepared using any sample (C ppm). A spike concentration (S ppm) was added to the sample as follows: 50 ppm of calcium, 25 ppm of potassium, and 80 ppm of sodium. The concentration of the prepared quality control samples was determined (Q ppm). The standards, quality control samples, and the samples of toothpaste solution were introduced to the flame photometer and the emitted wavelength was measured. The concentration of the quality control samples and the samples was determined from the created standard curves. The results of the samples were approved if the R2 of the standard curves was more than 0.98 and if the recovery percentage of the quality control samples was more than 75% [19].
The magnesium concentration was determined using the spectrophotometer (JENWAY 6850 UV/VIS) and the readymade kits of Human diagnostic company (Human diagnostics, Wiesbaden, Germany). The measurement was carried out at the wavelength of 520 nm [20].
Statistical analysis
The obtained results were statistically analyzed using the Analysis of Variance test (ANOVA) of the Statistical Package for Social Sciences (SPSS).
Results
As mentioned in the introduction section of this manuscript, the major constituents of the toothpastes are the fluoride, antibacterial, detergents, humectant, binder, flavoring, anti-calculus and anti-sensitivity compounds [21].
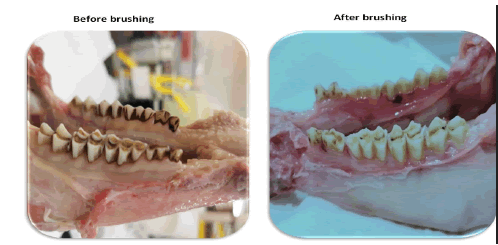
The brushing process whitened the jaws of the sheep possibly due to the effect of the brushing process and the chemical ingredients of the toothpaste solutions as shown in Figure 1.
Figure 1. Effect of brushing on the whitening of the teeth.
pH
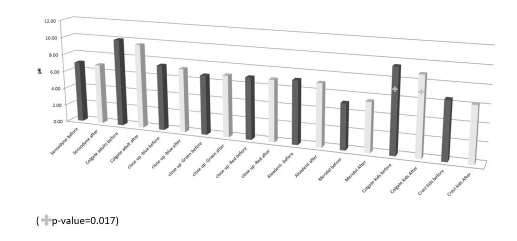
The mean ± SD and range of the pH of the toothpaste solutions were as follows: Sensodyne (7.0 ± 0.26; 6.7-7.2), Colgate adult ((10.03 ± 0.06; 10.0-10.1), Closeup blue (7.47 ± 1.15; 6.8-8.8), Closeup green (6.77 ± 0.06; 6.7- 6.8), Closeup red (7.0 ± 0.00; 7.0-7.0), Aloedent (7.17± 0.15; 7.0-7.3), Meridol (5.13 ± 0.06; 5.1- 5.3), Colgate kids (9.5 ± 0.00; 9.5-9.5) and crest kids (6.53 ± 0.06; 6.5-6.6). The pH results of the toothpaste solutions after jaw wash were Sensodyne (6.9 ± 0.1; 6.8-7.0), Colgate adult ((9.63 ± 0.15; 9.5-9.8), Closeup blue (7.3 ± 0.10; 7.2-7.4), Closeup green (7.0 ± 0.1; 6.9-7.1), Closeup red (7.0 ± 0.00; 7.0-7.0), Aloedent (7.07 ± 0.06; 7.0-7.1), Meridol (5.57 ± 0.12; 5.5-5.7), Colgate kids (8.9 ± 0.17; 8.7-9.0) and Crest kids (6.27 ± 0.06; 6.2-6.3).
The pH of the Colgate kids solution was significantly decreased in the after-brushing solution compared to the prebrushing toothpaste solution (Figure 2). The pH value of the after-brushing toothpastes solution was decreased in all the toothpastes solutions except the solutions of Closeup green (from 6.77 to 7.0) and Meridol (from 5.13 to 5.57).
Figure 2. Effect of toothpaste on the pH of the oral wash. The pH of the colgate kids toothpaste solution before oral washing was significantly more than the pH of its solution after oral washing.
Conductivity
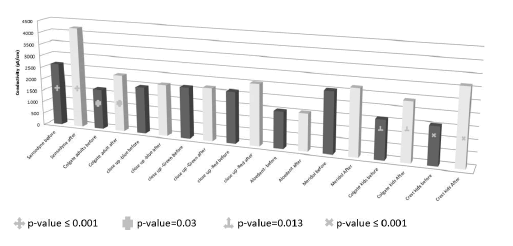
The mean ± SD and range of the conductivity (μS/cm) the Sensodyne before, Sensodyne after, Colgate adults before, Colgate adults after, Close up-blue before, Close up-blue after, Close up-green before, Close up-green after, Close up-red before, Close up-red after, Aloedent-before, Aloedent-after, Meridol before, Meridol after, Colgate kids before, Colgate kids after, Crest kids before and Crest kids after were (2636.7 ± 1024.8; 2040-3820), (4240 ± 271.8; 3940-4470), (1686.7 ± 190.1; 1500-1880), (2390 ± 270.7; 2080-2580), (1953.3 ± 66.6; 1910-2030), (2146.7 ± 73.7; 2090-2230), (2126.7 ± 5.8; 2120-2130), (2190 ± 20; 2170-2210), (2136.7 ± 28.9; 2120-2170), (2546.7 ± 80.8; 2460-2620), (1526.7 ± 851.7; 1020-2510), (1546.7 ± 28.9; 1530-1580), (2530 ± 20; 2510-2550), (2726.7 ± 15.3; 2710-2740), (1596.7 ± 72.3; 1550-1680), (2406.7 ± 526.5; 2040-3010), (1560 ± 580.4; 1210-2230) and (3150 ± 105.4; 3040-3250), respectively.
The conductivity of the after-brushing toothpaste solutions was more than that of before-brushing. The significant increase in the conductivity value was reported for Sensodyne (p-value ≤ 0.001), Colgate adults (p-value=0.03), Colgate kids (p-value=0.013) and Crest kids (p-value ≤ 0.001). The conclusion is that the Sensodyne, Colgate adults, Colgate kids and crest kids are the most effective toothpastes in the removal of tartar (Figure 3).
Figure 3. The mean conductivity values of the toothpaste solutions before and after the oral wash. The Sensodyne, Colgate adults, Colgate kids and crest kids toothpastes significantly increased the conductivity of the toothpaste solutions which reflected the effectiveness of these toothpastes in the removal of tartar.
TDS
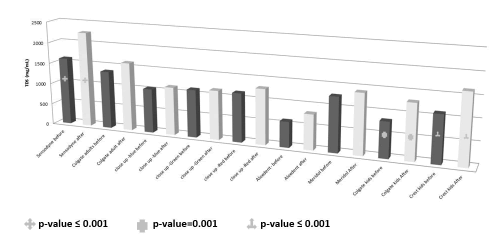
The results of the TDS (g/L) of the toothpastes solutions showed that the mean ± SD and range of Sensodyne before was (1603.3 ± 440.04; 1160-2040), Sensodyne after (2263.3 ± 196.6; 2040-2410), Colgate adults before (1366.7 ± 202.6; 1140-1530), Colgate adults after (1620 ± 345.1; 1270-1960), close up-blue before (1043.3 ± 23.1; 1030-1070), close up-blue after (1133.3 ± 40.4; 1110-1180), close up-green before (1123.3 ± 30.6; 1090-1150), close up green after (1156.7 ± 15.3; 1140-1170), close up-red before (1146.7 ± 15.3; 1130-1160), close up-red after (1300 ± 75.5; 1220-1370), Aloedent before (600 ± 95.4; 540-710), Aloedent after (826.7 ± 5.8; 820-830), Meridol before (1276.7 ± 23.1; 1250-1290), Meridol after (1413.3 ± 25.2; 1390-1440), Colgate kids before (833.3 ± 15.3; 820-850), Colgate kids after (1300 ± 260; 1140-1600), crest kids before (1110 ± 72.1; 1030-1170) and crest kids after (1643.3 ± 70.9; 1580-1720).
The TDS was significantly increased in the after- brushing toothpaste solutions of Sensodyne (p-value ≤ 0.001), Colgate kids (p-value=0.001) and crest kids (p-value ≤ 0.001) (Figure 4).
Figure 4. The TDS of the toothpaste solutions before and after the oral wash. The Sensodyne, Colgate kids and crest kids significantly increased the TDS value of their oral wash solutions.
Carbohydrates
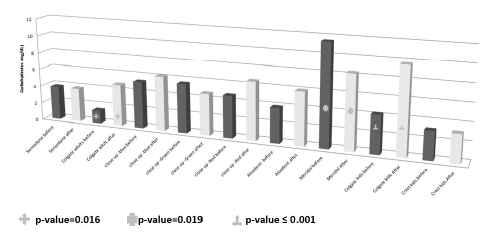
The mean, SD and range of the carbohydrates concentration (mg/dL) in the pre and after brushing toothpaste solutions are presented in Table 1. The carbohydrates concentration in the after brushing toothpaste solution was significantly increased compared to the pre brushing toothpaste solutions of Colgate adults and Colgate kids (pvalue= 0.016 and p-value ≤ 0.001, respectively). The carbohydrates concentration in the after brushing toothpaste solution of Meridol was significantly decreased compared to the carbohydrate concentration of the pre brushing solution (p-value=0.019) (Figure 5).
| Toothpaste | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
|
Carbohydrates Mg/dL |
Sensodyne before |
3.8474 |
0.84325 |
3.09 |
4.76 |
|
Sensodyne after |
3.8414 |
0.63273 |
3.41 |
4.57 |
|
|
Colgate adults before |
1.5041 |
0.53613 |
0.93 |
1.99 |
|
|
Colgate adult after |
4.7741 |
1.60523 |
3.27 |
6.46 |
|
|
Closeup-blue before |
5.3491 |
0.82508 |
4.52 |
6.17 |
|
|
Closeup-blue after |
6.1941 |
0.37096 |
5.8 |
6.53 |
|
|
Closeup-green before |
5.6008 |
0.21704 |
5.42 |
5.84 |
|
|
Closeup-green after |
4.7208 |
1.76501 |
2.73 |
6.1 |
|
|
Closeup-red before |
4.7508 |
0.59225 |
4.36 |
5.43 |
|
|
Closeup-red after |
6.5841 |
0.23153 |
6.32 |
6.76 |
|
|
Aloedent-before |
3.9391 |
1.16099 |
2.67 |
4.94 |
|
|
Aloedent after |
6.0008 |
1.33787 |
4.74 |
7.4 |
|
|
Meridol before |
11.5491 |
2.41057 |
9.71 |
14.28 |
|
|
Meridol after |
8.3724 |
0.32909 |
7.99 |
8.56 |
|
|
Colgate kids before |
4.2641 |
1.2199 |
3 |
5.44 |
|
|
Colgate kids after |
9.7291 |
5.08745 |
3.86 |
12.82 |
|
|
Crest kids before |
3.1691 |
0.43466 |
2.84 |
3.66 |
|
|
Crest kids after |
3.1641 |
0.29387 |
2.97 |
3.5 |
|
Table 1. Results of carbohydrates of pre and after brushing toothpaste solutions.
Figure 5. The carbohydrates concentration in the oral wash of the studied toothpastes. The effective toothpastes in the removal of the carbohydrates from the teeth are the Colgate adults and Colgate kids.
Total proteins
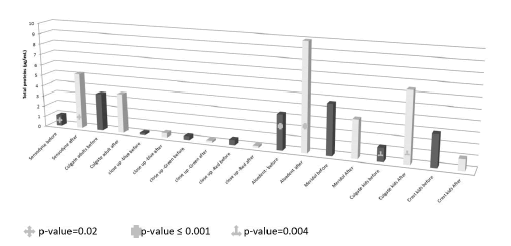
The mean, SD and range of the proteins concentration (μg/mL) in the pre and after brushing toothpaste solutions are presented in Table 2. The proteins concentration in the after brushing toothpaste solution was significantly increased compared to the pre brushing toothpaste solutions of Sensodyne, Aloedent and Colgate kids (p-value=0.02, p-value ≤ 0.001 and p-value=0.004, respectively). The proteins concentration in the after brushing toothpaste solution of Meridol and Crest kids was insignificantly decreased compared to the protein concentration of the pre brushing solution (Figure 6). The decrease of the protein concentration of the after brushing toothpaste solutions of Meridol and Crest kids may be due to the precipitation of the proteins on the surface of teeth because of their characteristic contents such as the hydrogenated castor oil and hydroxyethyl ammonium difluoride and stannous fluoride and 3-(NHexadecyl- N-2-hydroxyethylammonio) propylbis (meridol) and carbomer 956 (crest kids). The significant increase of the proteins in the after- brushing toothpaste solutions of Sensodyne, Aloedent and Colgate kids could be due to their effectiveness in the removal of dental plaque.
| Toothpaste | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Protein µg/mL | Sensodyne before | 1.0394 | 0.4389 | 0.78 | 1.55 |
| Sensodyne after | 5.2394 | 3.74996 | 2.79 | 9.56 | |
| Colgate adults before | 3.516 | 0.30116 | 3.26 | 3.85 | |
| Colgate adult after | 3.706 | 0.52849 | 3.15 | 4.2 | |
| Closeup-blue before | 0.1927 | 0.1193 | 0.06 | 0.28 | |
| Closeup-blue after | 0.4227 | 0.10017 | 0.35 | 0.54 | |
| Closeup-green before | 0.356 | 0.38223 | 0.11 | 0.8 | |
| Closeup-green after | 0.186 | 0.14422 | 0.07 | 0.35 | |
| Closeup-red before | 0.506 | 0.26287 | 0.21 | 0.7 | |
| Closeup-red after | 0.1827 | 0.12897 | 0.08 | 0.33 | |
| Aloedent-before | 3.3294 | 3.95611 | 0.95 | 7.9 | |
| Aloedent after | 9.9527 | 1.08565 | 8.85 | 11.02 | |
| Meridol before | 4.6894 | 1.57348 | 3.76 | 6.51 | |
| Meridol after | 3.5427 | 0.53295 | 3.14 | 4.15 | |
| Colgate kids before | 1.266 | 0.49568 | 0.76 | 1.75 | |
| Colgate kids after | 6.4994 | 5.05206 | 2.47 | 12.17 | |
| Crest kids before | 2.9627 | 4.40237 | 0.4 | 8.05 | |
| Crest kids after | 1.046 | 0.44508 | 0.74 | 1.56 | |
Table 2. The protein concentration in the pre and after brushing toothpaste solutions.
Figure 6. The protein concentration of the toothpaste solutions before and after the oral wash. The effective toothpastes in the removal of proteins from the teeth surface are the Synsodyne, Aloedent and Colgate kids.
Sodium
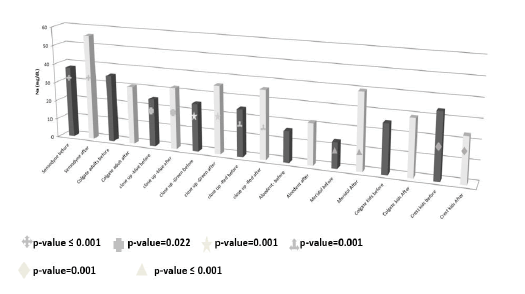
The means, SD and range of the sodium concentration (mg/dL) of the pre and after brushing toothpaste solution are presented in Table 3. The concentration of the sodium was significantly increased in the after-brushing solutions of the Sensodyne, Closeup blue, Closeup green, Closeup red and Meridol while it was significantly decreased in the after-brushing solution of crest kids toothpaste which may be due to its content of carbomer 956 (Figure 7). Moreover, there was insignificant decrease in the sodium concentration of the after-brushing solution of Colgate adult toothpaste.
| Toothpaste | Mean | SD | Minimum | Maximum | |
| Na mg/dL | Sensodyne before | 37.7333 | 8.807 | 31 | 47.7 |
| Sensodyne after | 55.8333 | 2.05264 | 54.1 | 58.1 | |
| Colgate adults before | 35.4 | 2.45561 | 32.7 | 37.5 | |
| Colgate adult after | 30.7 | 1.63707 | 28.9 | 32.1 | |
| Closeup-blue before | 25.1667 | 0.05774 | 25.1 | 25.2 | |
| Closeup-blue after | 32.3 | 2.17025 | 30.9 | 34.8 | |
| Closeup-green before | 25.1333 | 0.9815 | 24 | 25.7 | |
| Closeup-green after | 35.7667 | 2.02073 | 33.6 | 37.6 | |
| Closeup-red before | 24.7667 | 1.42945 | 23.2 | 26 | |
| Closeup-red after | 36 | 2.52389 | 33.2 | 38.1 | |
| Aloedent-before | 16.3333 | 1.25033 | 15.1 | 17.6 | |
| Aloedent after | 21.7 | 0.65574 | 21 | 22.3 | |
| Meridol before | 13.4333 | 1.85831 | 11.3 | 14.7 | |
| Meridol after | 39.7 | 11.19062 | 26.8 | 46.8 | |
| Colgate kids before | 25.6 | 1.22882 | 24.2 | 26.5 | |
| Colgate kids after | 29.5 | 0 | 29.5 | 29.5 | |
| Crest kids before | 33.9333 | 0.15275 | 33.8 | 34.1 | |
| Crest kids after | 23.5 | 0 | 23.5 | 23.5 | |
Table 3. The results of the sodium concentration in the pre and after brushing toothpaste solutions.
Figure 7. Sodium concentration in the tooth paste solutions before and after teeth wash.
Potassium
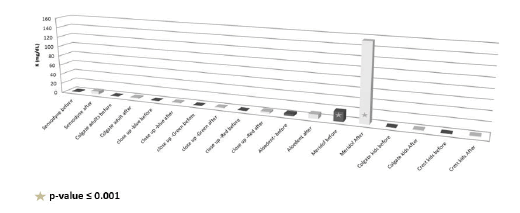
The mean, SD, minimum and maximum values of the potassium concentration in the pre and after-brushing solution of the studied toothpastes are presented in Table 4. The after-brushing toothpaste solution of meridol was characterized by significantly increased potassium concentration (p-value ≤ 0.001) (Figure 8).
| Toothpaste | Mean | SD | Minimum | Maximum | |
| K mg/dL | Sensodyne before | 1 | 0.75498 | 0.3 | 1.8 |
| Sensodyne after | 6.1333 | 6.9515 | 1.3 | 14.1 | |
| Colgate adults before | 0.4 | 0.17321 | 0.3 | 0.6 | |
| Colgate adult after | 1.9 | 0.7 | 1.4 | 2.7 | |
| Closeup-blue before | 0.2333 | 0.05774 | 0.2 | 0.3 | |
| Closeup-blue after | 1.2333 | 0.65064 | 0.6 | 1.9 | |
| Closeup-green before | 0.1 | 0 | 0.1 | 0.1 | |
| Closeup-green after | 1.4 | 1.56205 | 0.4 | 3.2 | |
| Closeup-red before | 0.1 | 0 | 0.1 | 0.1 | |
| Closeup-red after | 3.3667 | 2.97041 | 1 | 6.7 | |
| Aloedent-before | 4 | 0.60828 | 3.3 | 4.4 | |
| Aloedent after | 8.9667 | 1.42244 | 8 | 10.6 | |
| Meridol before | 19.0333 | 2.77549 | 16.5 | 22 | |
| Meridol after | 155.0667 | 131.5204 | 3.2 | 231 | |
| Colgate kids before | 0.1 | 0 | 0.1 | 0.1 | |
| Colgate kids after | 1.2 | 0 | 1.2 | 1.2 | |
| Crest kids before | 0.2 | 0 | 0.2 | 0.2 | |
| Crest kids after | 1.8333 | 1.40475 | 0.5 | 3.3 | |
Table 4. The results of the potassium concentration in the pre and after brushing toothpaste solutions.
Figure 8. Potassium concentrations in the toothpaste solutions before and after teeth wash.
Calcium
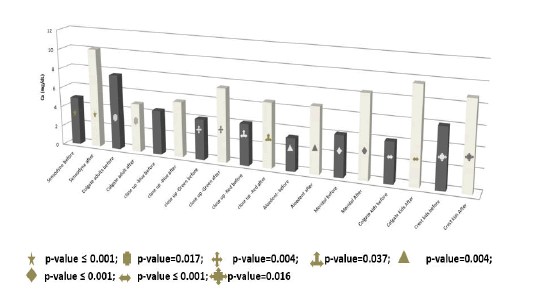
The means, SD and range of the calcium concentration in the pre and after-brushing toothpaste solutions are presented in Table 5. The calcium concentration increased in all the after-brushing toothpaste solutions except in that of Colgate adults which was significantly decreased (p-value=0.017). The significant decrease in the calcium concentration of the after-brushing solution of colgate adults, reflects its re-mineralization efficiency. The calcium concentration was significantly increased in the after-brushing solutions of sensodyne (p-value ≤ 0.001), Closeup green (p-value=0.004), Closeup red (p-value=0.037), Aloedent (p-value=0.004), meridol (p-value ≤ 0.001), colgate kids (p-value ≤ 0.001) and crest kids (p-value=0.016) (Figure 9). The significant increase of the calcium concentration in the after-brushing toothpaste solution shows the effectiveness of these toothpastes in the removal of tartar.
| Toothpaste | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Ca mg/dL | Sensodyne before | 4.9 | 0.8544 | 4.1 | 5.8 |
| Sensodyne after | 10.1333 | 0.75056 | 9.7 | 11 | |
| Colgate adults before | 7.6667 | 2.55408 | 5.2 | 10.3 | |
| Colgate adult after | 4.9333 | 0.23094 | 4.8 | 5.2 | |
| Closeup-blue before | 4.4667 | 0.30551 | 4.2 | 4.8 | |
| Closeup-blue after | 5.6333 | 0.60277 | 5 | 6.2 | |
| Closeup-green before | 4.1 | 0.17321 | 3.9 | 4.2 | |
| Closeup-green after | 7.4667 | 0.63509 | 7.1 | 8.2 | |
| Closeup-red before | 4.1667 | 0.05774 | 4.1 | 4.2 | |
| Closeup-red after | 6.5333 | 1.04083 | 5.7 | 7.7 | |
| Aloedent-before | 3.3 | 0.17321 | 3.2 | 3.5 | |
| Aloedent after | 6.6333 | 1.36504 | 5.7 | 8.2 | |
| Meridol before | 4.1333 | 1.62891 | 3 | 6 | |
| Meridol after | 8.3667 | 3.34863 | 4.5 | 10.3 | |
| Colgate kids before | 4.0667 | 0.28868 | 3.9 | 4.4 | |
| Colgate kids after | 9.6333 | 1.98578 | 8.2 | 11.9 | |
| Crest kids before | 6.0333 | 1.10151 | 5.3 | 7.3 | |
| Crest kids after | 8.8 | 1.21655 | 8 | 10.2 | |
Table 5. The results of the calcium concentration in the pre and after-brushing toothpaste solutions.
Figure 9. Calcium concentration in the toothpaste solutions before and after teeth wash.
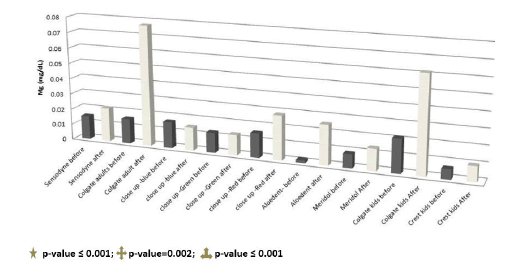
Magnesium
Table 6 shows the results of the magnesium concentration in the pre and after-brushing solutions of the different toothpastes expressed as mean, SD and range. The magnesium concentration was significantly increased in the afterbrushing solutions of Colgate adults (p-value ≤ 0.001), Aloedent (p-value=0.002) and Colgate kids (p-value ≤ 0.001) (Figure 10).
|
Toothpaste |
Mean |
SD |
Minimum |
Maximum |
|
|
Mg mg/mL |
Sensodyne before |
0.015 |
0.00868 |
0.01 |
0.02 |
|
Sensodyne after |
0.0216 |
0.00196 |
0.02 |
0.02 |
|
|
Colgate adults before |
0.0159 |
0.01129 |
0 |
0.02 |
|
|
Colgate adult after |
0.0772 |
0.00719 |
0.07 |
0.08 |
|
|
Closeup-blue before |
0.0168 |
0.00626 |
0.01 |
0.02 |
|
|
Closeup-blue after |
0.0148 |
0.00228 |
0.01 |
0.02 |
|
|
Closeup-green before |
0.0128 |
0.00434 |
0.01 |
0.02 |
|
|
Closeup-green after |
0.013 |
0.005 |
0.01 |
0.02 |
|
|
Closeup-red before |
0.0155 |
0.01027 |
0.01 |
0.03 |
|
|
Closeup-red after |
0.0283 |
0.01495 |
0.02 |
0.05 |
|
|
Aloedent-before |
0.0017 |
0.00245 |
0 |
0 |
|
|
Aloedent after |
0.0254 |
0.00857 |
0.02 |
0.03 |
|
|
Meridol before |
0.0088 |
0.00222 |
0.01 |
0.01 |
|
|
Meridol after |
0.0138 |
0.00185 |
0.01 |
0.02 |
|
|
Colgate kids before |
0.0214 |
0.01938 |
0.01 |
0.04 |
|
|
Colgate kids after |
0.0616 |
0.01585 |
0.05 |
0.08 |
|
|
Crest kids before |
0.0063 |
0.00098 |
0.01 |
0.01 |
|
|
Crest kids after |
0.0098 |
0.00097 |
0.01 |
0.01 |
|
Table 6. The results of the magnesium concentration in the pre and after-brushing toothpaste solutions.
Figure 10. Magnesium concentration in the toothpaste solutions before and after teeth wash.
Discussion
The decrease in the pH of the after-brushing toothpaste may be due to the depletion of the acids from the teeth surfaces. Dehghan, et al. studied the effect of listerine oral wash on the saliva pH after exposure to acidic juice and concluded that the oral wash significantly increased the pH of the saliva probably through removal of acids from the saliva [22]. The increase in the pH of the after-brushing toothpaste solution of Closeup green and Meridol could be referred to demineralization of teeth due to the low pH of the toothpaste solutions (6.77 for Closeup green and 5.13 for Meridol) [23-25]. Delgado, et al. found that tooth structure lost was associated with the acidic oral moisturizers rather than the neutral oral moisturizers [26].
Omar, et al. measured the conductivity of different toothpaste solutions including Colgate and crest and reported a range of conductivity of (Ë?10-30 μS/cm) [27]. The conductivity value of Omar, et al. is far less than the conductivity of the toothpastes solutions of this study (1210-4470 μS/cm). We could not find any study which investigated the effect of brushing on the conductivity of the after-brushing solutions.
Carbohydrates concentration in all the toothpastes is reported to be of zero concentration. However, it is known that the thickening materials of the toothpastes are either natural gums or polysaccharides such as cellulose, cellulose derivatives and carrageenan [5]. Natural gums such as the xanthan gum are composed of polysaccharide polymers beside traces of monosaccharide molecules such as arabinose, galactose, glucose, mannose and xylose [28-31]. The presence of sugars in the pre-brushing toothpaste solutions may be due to the presence of the thickening materials. Regarding the concentration of the carbohydrates in the after brushing solutions, this is the first study to report that toothpastes can release the carbohydrate part of the plaque in the after brushing solution. The after brushing solution of meridol toothpaste was characterized by significantly low carbohydrates concentration compared to the prebrushing solution. This may be due to attachment of some sugars to the teeth because of its content of hydrogenated castor oil or the hydroxyethyl) ammonium difluoride and Stannous Fluoride.
The protein measurement of the toothpaste solutions showed that they contain trace amounts of proteins which may be due to the natural gums that used as binding materials [28-32].
Sodium is well known to be present in the toothpaste constituents as sodium fluoride, sodium Alginate (binder), sodium carboxym-ethylcellulose (CMC) (binder), sodium metaphosphate (abrasive), Sodium lauryl sulphate (SLS) (foaming agent) [31]. The increased amount of sodium in the after-brushing toothpaste solutions may be referred to the effect of abrasive chemicals in the toothpaste which may release the sodium content of the hard dentin enamel [33]. The tartar contains calcium, phosphate, fluoride, carbonate, silicon and magnesium and has no sodium in its structure, therefore the increased sodium concentration in the after-brushing solutions is not from the tartar [34].
The potassium is added to the toothpastes in the forms of potassium nitrate as an anti-sensitivity agent and tetrapotassium pyrophosphate as anti-calculus [35,36]. As mentioned before, the tartar is composed of calcium salts, fluoride, carbonate, magnesium and silicon, it has no potassium in its structure [34]. The increased potassium concentration in the after-brushing solution of the meridol may be due to its release from the teeth enamel, because of the effects of brushing or abrasives action [37].
Toothpastes contain calcium in different forms and functions such as calcium hydrogen phosphate and calcium carbonate (Abrasives) and calcium phosphate as re-mineralizer.3,5 calcium is the major constituent of tartar and dental enamel [34,37]. It is expected that its concentration increases in the after-brushing solutions of toothpastes.
Magnesium is added to the toothpastes in the form of magnesium carbonate which acts as abrasive material. It is expected that the magnesium concentration increases in the after-brushing solution of toothpastes because the tartar and the dental enamel contain some magnesium concentration.
Conclusion
In conclusion, the toothpastes exerted variable significant effects on the studied parameters which may be due to the different chemical constituents. Colgate kidsâ?? toothpaste was the most effective in the removal of plaque and tartar followed by Sensodyne and Colgate adults. The Colgate adult is the best in sodium and calcium re-mineralization.Acknowledgement
The authors extend their appreciation to the poison control and medical forensic chemistry centre, ministry of health, Asir region, Saudi Arabia for their technical assistance.Funding Information
This research was funded through small group research project of the deanship of scientific research at King Khalid University-grant number 44/494. Moreover, this research was logistically supported by the Saudi Aramco.Conflict of Interest
The authors declare no conflict of interest.Authors' Contributions
The authors contributed equally for the preparation of this manuscript as follows: Laheq A, Brima EI and Mohammed MEA designed the research. Laheg A and Alasmari AD and Sahlabji T did the practical work. Laheg A and Mohammed MEA did the statistical analysis. The Manuscript was drafted by Laheg A and revised by Mohammed MEA, Brima EI and Alasmari AD. All authors read and approved the final version of the manuscript.References
- Tiwari S, et al. A Comparative Study of Toothpaste Available in Same Brand: A Physio-Chemical Analysis. Journal of Pharmaceutical Sciences and Research, Vol. 12, No. 12, 2020, pp. 1510-1514.
- Lippert F. An introduction to toothpaste-its purpose, history and ingredients. InToothpastes, Vol. 23, 2013, pp. 1-14.
[Crossref] [Google Scholar] [PubMed]
- Moharamzadeh K. Biocompatibility of oral care products. InBiocompatibility of Dental Biomaterials, 2017, pp. 113-129.
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for fluoride. EFSA Journal, Vol. 11, No. 8, 2013, pp. 3332.
- Reynolds EC. Contents of toothpaste-safety implications. Australian Prescriber, Vol. 17, No. 2, 1994, pp. 49-51.
- Listgarten MA. The structure of dental plaque. Periodontology 2000, Vol. 5, No. 1, 1994, pp. 52-65.
[Crossref] [Google Scholar] [PubMed]
- Rudney JD. Saliva, and dental plaque. Advances in Dental Research, Vol. 14, No. 1, 2000, pp. 29-39.
[Crossref] [Google Scholar] [PubMed]
- de Sousa-Pereira P, and Woof JM. IgA: Structure, function, and developability. Antibodies, Vol. 8, No. 4, 2019, pp. 57.
[Crossref] [Google Scholar] [PubMed]
- Okpalugo J, Ibrahim K, and Inyang US. Toothpaste formulation efficacy in reducing oral flora. Tropical Journal of Pharmaceutical Research, Vol. 8, No. 1, 2009, pp. 71-77.
- Abraham JA, et al. A crystallinity study of dental tissues and tartar by infrared spectroscopy. Analytical and Bioanalytical Chemistry, Vol. 399, 2011, pp. 1699-1704.
[Crossref] [Google Scholar] [PubMed]
- White DJ. Dental calculus: Recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. European Journal of Oral Sciences, Vol. 105, No. 5, 1997, pp. 508-522.
[Crossref] [Google Scholar] [PubMed]
- Forshaw R. Dental calculus-oral health, forensic studies and archaeology: A review. British Dental Journal, Vol. 233, No. 11, 2022, pp. 961-967.
[Crossref] [Google Scholar] [PubMed]
- Eke PI, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research, Vol. 91, No. 10, 2012, pp. 914-920.
[Crossref] [Google Scholar] [PubMed]
- Ciancio SG. Baking soda dentifrices and oral health. The Journal of the American Dental Association, Vol. 148, No. 11, 2017, pp. S1-3.
[Crossref] [Google Scholar] [PubMed]
- Gallagher A, et al. The effect of brushing time and dentifrice on dental plaque removal in vivo. American Dental Hygienists' Association, Vol. 83, No. 3, 2009, pp. 111-116.
[Google Scholar] [PubMed]
- Eftekhardadkhah M and Hashemabadi SH. Influence of Salinity, Surfactants and Power of Ultrasonic Homogenizer on Droplet Size Distribution of Crude Oil/Water Emulsions. Iranian Journal of Chemical Engineering, Vol. 8, No. 3, 2011, pp. 55-63.
- Ludwig TG, and Goldberg HJ. The anthrone method for the determination of carbohydrates in foods and in oral rinsing. Journal of Dental Research, Vol. 35, No. 1, 1956, pp. 90-94.
[Crossref] [Google Scholar] [PubMed]
- Azeredo LD, et al. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chemistry, Vol. 80, No. 2, 2003, pp. 249-254.
[Google Scholar] [PubMed]
- Singh M, et al. Quantification of minerals and trace elements in raw caprine milk using flame atomic absorption spectrophotometry and flame photometry. Journal of Food Science and Technology, Vol. 52, 2015, pp. 5299-5304.
[Crossref] [Google Scholar] [PubMed]
- Hadzimusic N, Krnıä J. Values of calcium, phosphorus and magnesium concentrations in blood plasma of cows in dependence on the reproductive cycle and season. Ä°stanbul Üniversitesi Veteriner Fakültesi Dergisi, Vol. 38, No. 1, 2012, pp. 1-8.
- Subramanian S, et al. The role of abrasives in dentifrices. Journal of Pharmaceutical Sciences and Research, Vol. 9, No. 2, 2017, pp. 221.
- Dehghan M, et al. Neutralizing salivary pH by mouthwashes after an acidic challenge. Journal of Investigative and Clinical Dentistry, Vol. 8, No. 2, 2017, pp. e12198.
[Crossref] [Google Scholar] [PubMed]
- Bellini HT, Arneberg P, and Von Der Fehr FR. Oral hygiene and caries: A review. Acta Odontologica Scandinavica, Vol. 39, No. 5, 1981, pp. 257-265.
[Crossref] [Google Scholar] [PubMed]
- Hara AT, et al. Interplay between fluoride and abrasivity of dentifrices on dental erosion–abrasion. Journal of Dentistry, Vol. 37, No. 10, 2009, pp. 781-785.
[Crossref] [Google Scholar] [PubMed]
- McComas M. Dental hygienists should be prepared to advise patients about the role pH neutralization plays in maintaining oral health. Dimensions of Dental Hygiene, Vol. 17, No. 8, 2019, pp. 11.
- Delgado AJ, Olafsson VG, and Donovan TE. pH and erosive potential of commonly used oral moisturizers. Journal of Prosthodontics, Vol. 25, No. 1, 2016, pp. 39-43.
[Crossref] [Google Scholar] [PubMed]
- Omar KA, et al. Physico-Chemical characteristics of toothpastes and natural powder and study their antibacterial activity against Viridans streptococci Bacteria. Oriental Journal of Chemistry, Vol. 33, No. 5, 2017, pp. 2566.
- Murthy HN. Chemical constituents and applications of gums, resins, and latexes of plant origin. Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses, Vol. 11, 2021, pp. 1-21.
- Mirhosseini H and Amid BT. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Research International, Vol. 46, No. 1, 2012, pp. 387-398.
- Barak S, Mudgil D, and Taneja S. Exudate gums: chemistry, properties and food applications–a review. Journal of the Science of Food and Agriculture, Vol. 100, No. 7, 2020, pp. 2828-2835.
[Crossref] [Google Scholar] [PubMed]
- Vranic E, et al. Formulation ingredients for toothpastes and mouthwashes. Bosnian Journal of Basic Medical Sciences, Vol. 4, No. 4, 2004, pp. 51.
[Crossref] [Google Scholar] [PubMed]
- Hamdani AM, Wani IA and Bhat NA. Sources, structure, properties and health benefits of plant gums: A review. International Journal of Biological Macromolecules, Vol. 135, No. 2019, pp. 46-61.
[Crossref] [Google Scholar] [PubMed]
- Tanaskovic Stankovic S, et al. The mineral content of the hard dental tissue of mesiodens. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc, Vol. 162, No. 2, 2018, pp. 149-153.
[Crossref] [Google Scholar] [PubMed]
- Nozaki K, et al. Tartar and plaque control. InMineral Scales and Deposits, 2015, pp. 353-372.
- Venkataramana S, et al. Comparative evaluation of the efficacy of three commercially available toothpastes on dentin hypersensitivity reduction: An 8-week clinical study. Indian Journal of Dental Sciences, Vol. 12, No. 2, 2020, pp. 87-91.
- de Lattre VF. Factors contributing to adverse soft tissue reactions due to the use of tartar control toothpastes: Report of a case and literature review. Journal of Periodontology, Vol. 70, No. 7, 1999, pp. 803-807.
[Crossref] [Google Scholar] [PubMed]
- Zipkin I. The inorganic composition of bones and teeth. InBiological Calcification: Cellular and Molecular Aspects, 1970, pp. 69-103.