Research - International Journal of Medical Research & Health Sciences ( 2021) Volume 10, Issue 5
Exploring Potential Intervention of Convalescent Plasma-Derived Hyper-immunized Antibodies to Combat COVID-19: A Mini-Review
Viveeyan Saikia1 and Archana Deka2*2ICMR-Regional Medical Research Centre, N.E. Region, Dibrugarh, Assam, India
Archana Deka, ICMR-Regional Medical Research Centre, N.E. Region, Dibrugarh, Assam, India, Email: archanadeka001@gmail.com
Received: 21-Apr-2021 Accepted Date: May 18, 2021 ; Published: 25-May-2021
Abstract
Globally, COVID-19 caused by SARS-CoV-2 has resulted in millions of infections and several thousand deaths. Amid the race for the development of vaccines and antiviral drugs, convalescent plasma therapy has emerged as an emergency treatment for SARS-CoV-2 infected patients. In this review, we provide an overview of the use of passive immunization-based therapy as an immediate measure during novel viral infections when no other treatments are available. We also discuss the benefits and challenges faced by the use of convalescent plasma therapy in the COVID-19 pandemic. Further, we attempted to stress the relevance of convalescent plasma-derived hyper-immunized immunoglobulins with high neutralizing activity as a potential therapeutic intervention for COVID-19.
Keywords
Hyper-immunized immunoglobulins, Convalescent plasma, COVID-19, SARS-CoV-2, Passive immunity
Introduction
The use of passive immunization or immunotherapy was first introduced by Emil von Behring and Shibasaburo Kitasato in the year 1890 for treating diphtheria and tetanus [1,2]. In their experiments, an inactivated form of Clostridium tetani (causative agent of tetanus) and Corynebacterium diphtheria (causative agent of diphtheria) were injected into animals and whole blood was collected from these animals. Upon administration of blood to a different set of experimental animals, immunity towards the causative agents of tetanus and diphtheria was developed. They proposed the presence of mediators in the blood that can recognize and neutralize the foreign agents. Later, these mediators were referred to as antibodies. Antibodies (Abs) are proteins produced by the host’s immune system to fight against invasion of antigen or foreign agents. They help the body to recognize and thwart future attacks by the same antigen. Thus, passive immunotherapy involves the direct transfer of foreign antibodies to infected individuals that will provide temporary protection against infectious agents. It is a type of short-term immunization acquired against infectious agents by injecting pathogen-specific antibodies. Implementation of passive immunization resulted in the treatment of several infectious diseases (measles, rabies, hepatitis B, botulism, etc.) for which vaccines could not be developed [2,3]. The concept of passive immunotherapy also laid the foundation for anti-venom therapies used for the treatment of venomous bites caused by snakes and scorpions [4]. Anti-venoms are produced by fractionation of plasma obtained from large mammals hyper-immunized with snake venom. The most commonly used mammals for anti-venom production are horses and sheep [5-8]. After repeated immunization with venom to host animals, antibody response against different venom components is generated. The serum-containing antibody molecules (IgG) are collected and purified using various techniques such as precipitation or chromatography. First developed by Calmette, Phisalix, and Bertrand in 1894, plasma-derived anti-venoms remain the only treatment for victims of venomous bites.
In this mini-review, we discuss different aspects of convalescent plasma-based therapy, its usage as a promising rescue option for COVID-19 infections, and the challenges that need to be taken into consideration. Further, we explore the utility of hyper-immunized immunoglobulins from convalescent plasma for the treatment of serious viral infections including COVID-19. For the present study, we conducted a systematic search of the PubMed database using “convalescent plasma therapy” or “hyper-immunized immunoglobulins” and “COVID-19” as keywords, with no date limits.
More than 300 research articles were initially generated in the search, out of which 50 met our criteria for inclusion in the present review.
Passive Immunization and Convalescent Plasma Therapy
Convalescent plasma therapy, classic passive immunotherapy, has been applied as a potential treatment strategy in the previous epidemics caused by viral pathogens [9-11]. Convalescent Plasma (CP) contains a high concentration of neutralizing antibodies (blood proteins) and is collected from individuals who have recovered from the disease. Transfusion of these antibodies into freshly infected patients provides short-term humoral immunity, enhances their immune systems, and increases survival rates. During the SARS outbreak in 2003, seriously ill patients in Hong Kong and Taiwan were reportedly treated with convalescent serum obtained from individuals with SARS [12,13]. Reportedly, treatment with convalescent plasma also reduced the mortality in patients infected with influenza A virus (H1N1 2009) [14,15]. High incidences of deaths and ineffective therapy also led to the introduction of convalescent serum when MERS (Middle East Respiratory Syndrome) epidemic was triggered in 2012 [16,17]. Following the recommendation of the World Health Organization (WHO), the use of convalescent plasma was also attempted during the recent Ebola Virus Disease (EVD) outbreak [18]. Plasma immunotherapy has been the preferred therapeutic approach in these pandemics since it was safe and to some extent effective.
In the wake of a recent pandemic caused by a novel coronavirus (SARS-CoV-2), convalescent plasma therapy also gained spurred interest as an emerging treatment [19,20]. One of the reasons for its efficacy could be due to the presence of neutralizing antibodies specific to the novel coronavirus. Substantial evidence emphasized that COVID-19 patients produce SARS-Cov-2 specific antibodies within 2-3 weeks following the onset of symptoms [21,22]. It is a well-known fact that the entry of the coronavirus into the human cells is aided by spike protein present on the surface of the virus particle. These surface proteins appear to bind tightly to receptors on human cells. The spike protein is known to be immunogenic, meaning it can stimulate an immune response in the human body and consequently generate specific antibodies. As such, the antibodies generated can neutralize the virus and eventually prevent the disease [23]. Henceforth, blood plasma containing virus-specific antibodies could be opted as a valid option to control the severity of coronavirus infections.
For procurement of convalescent plasma for its use in the treatment of infections, utmost care needs to be taken [24]. To have a high concentration of neutralizing antibodies in the convalescent plasma, collection of blood from recovered patients should be done at least 3 weeks of post-infection. It is essential to note that convalescent plasma need to be transfused within days of collection and it is likely to be more beneficial for the treatment of early-stage infection. Before infusion of convalescent plasma, a thorough screening of blood for the presence of any infectious agents, blood type compatibility between donor and receiver are crucial. The immunity developed in the patient receiving convalescent plasma is usually short-term and the foreign antibodies present are often cleared from the recipient’s body within 30 days of administration. Since the recipient lacks its antibodies, the individuals are still susceptible to infection. Recipients seldom experience life-threatening complications following the procedure.
The plasma therapy showed favorable outcomes in preliminary studies carried out on critically ill patients with COVID- 19 [25,26]. A timely descriptive study was carried out to address the effectiveness of convalescent plasma on COVID-19 patients in Wuhan [27]. Results from this study emphasized the significance of convalescent plasma therapy during the COVID-19 crisis. In another study, researchers obtained convalescent plasma from individuals who recovered from COVID-19 and administered plasma to 10 critically ill patients [28]. All the patients receiving plasma therapy observed suppression of viremia (presence of virus in the blood) and eventually, all of them recovered [29]. Taken together, the outcomes were significant and sheds light on the potential benefits of convalescent therapy against COVID-19. Nevertheless, convalescent plasma therapy as a treatment of choice for COVID-19 is still in the nascent stage. Piechotta and colleagues provided a glimpse of different studies carried out to assess the efficacy of convalescent plasma therapy as COVID-19 treatment [30]. Taking into account the variable outcomes, the authors couldn’t ascertain whether convalescent therapy is beneficial for people with COVID-19. Hence, more randomized clinical trials, unbiased and well-designed studies are warranted to underscore the efficacy, safety, and risks associated with convalescent therapy in the treatment of COVID-19.
Despite the positive aspects of convalescent plasma therapy, a range of drawbacks prevent it from being used for COVID-19. The administration of convalescent plasma to patients with cardiovascular disorders and the elderly has been linked to the onset of serious allergic reactions, transfusion-related acute lung injury, and life-threatening bronchospasm [31-33]. In addition, a transient rise in body temperatures, mild fever, serum sickness, and anaphylaxis within hours of plasma transfusion has been observed [34,35]. Such complications can further worsen the respiratory symptoms of COVID-19 positive patients. The possibility of transmission of infectious agents such as human immunodeficiency virus, syphilis, hepatitis B virus, and hepatitis C virus further limits the application of convalescent plasma therapy [36]. It is an uncommon treatment option in the current pandemic due to the requirement of high plasma infusion volumes and donor compatibility with the recipient’s blood type. Further, no standard dose has been set for plasma infusion in recipients, and usage of varying doses has been reported in different studies [25,27,28,37]. Moreover, not all patients who have recovered from SARS-CoV-2 infection have the appropriate levels of antibodies for plasma donation. Another significant hindrance may be obtaining written informed consent from patients who have recently recovered from COVID-19 to donate plasma. Meanwhile, the ratio of cases being recovered from COVID-19 is far too less compared to those patients who need plasma. Given these limitations, convalescent plasma therapy has not gained desired attention in the current pandemic.
Hyper-immune Globulins from Pooled Convalescent Plasma as a Treatment for Viral Diseases
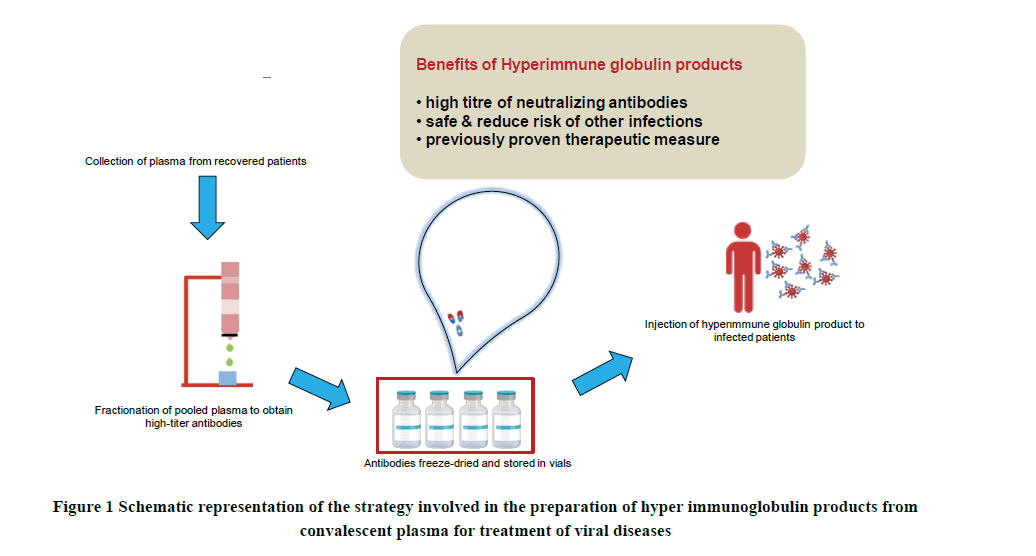
Inconsistent levels of virus-specific neutralizing antibodies in convalescent plasma may limit their use as a treatment for viral diseases. In contrast, more concentrated and purified neutralizing antibodies obtained from multiple donors offer several advantages over the whole plasma. The main advantage of using concentrated immunoglobulins is the high titer of neutralizing antibodies. Such a concentrated form of immunoglobulins will provide better specificity and potency. Moreover, highly concentrated antibodies with lower volumes will be administered to patients. A proper manufacturing infrastructure is an essential requirement for obtaining hyper immunoglobulin from convalescent plasma. Before assessment of plasma for potency, virus inactivation and its removal are performed to ensure the safety of the final product. The high-antibody titer is primarily tested by the Enzyme-Linked Immunosorbent Assay (ELISA), Immunofluorescence Assay, or Indirect Fluorescent Antibody (IFA) technique. This is followed by pooling or concentrating these antibodies and their purification. The purified antibodies are incubated in batches for several weeks to attain safe and efficient antibodies. The resultant antibodies are lyophilized and stored in a vial making it easy to distribute and administer to patients (Figure 1). Such hyper-immune globulin products reduce the risk of side effects caused by unwanted serum components and transmission of unwanted infectious agents.
Zhang and co-workers applied a combination of cold ethanol precipitation and ion-exchange chromatography to prepare SARS hyper-immune globulins for small-scale production [38]. In this study, virus-free hyper-immune globulins from SARS convalescent plasma were prepared by cold ethanol precipitation approach while anion-exchange chromatography and nanofiltration were performed to yield high-antibody titers. The affinity-chromatography approach for fractionation of plasma is particularly advantageous as it gives a better yield of hyper-immune globulins [39]. In industrialized countries, manufacturers of immunoglobulin products generally use highly sophisticated facilities and well-regulated technologies. Simple, cost-effective, and easy-to-use technologies are practical requirements for the preparation of hyper-immune immunoglobulin during infectious outbreaks in developing countries. A mini-pool IgG fractionation process based on caprylic acid precipitation is under development in Egypt [40]. Fractionation agents such as caprylic acid help in the purification of antibodies and retain their conformational stability. Such an easy-toimplement approach that uses disposable equipment (blood bags, hemodialyzer, filters) requires a small production area for isolation of IgG.
The use of hyper-immune immunoglobulins provides a new perspective in the prevention and treatment of infectious diseases, particularly those for which there is no such confirmed treatment [41,42]. A prospective multi-center randomized trial was carried out by Hung and colleagues to assess the antiviral activity of hyper-immune immunoglobulin fractionated from convalescent plasma for treatment of patients with severe A (H1N1) infection [43]. The outcome of the result inferred that hyper-immune immunoglobulin effectively suppressed viral load and reduced mortality in tested groups. Hyper-immunized immunoglobulins have shown initial signs of effectiveness in the treatment of SARS-CoV-1 infections. Based on the previous experience, the focus has been shifted from convalescent plasma therapy to plasma-derived immunoglobulins for the treatment of infection caused by SARS-CoV-2. Structural analysis on antibodies isolated from convalescent COVID-19 patients has shown that Spike (S) proteins of SARS-CoV-2 are widely identified as immunogens and potently neutralized by human antibodies [44,45]. For large-scale production and continuous supply, industries and companies globally have collaborated in an attempt to develop convalescent plasmaderived hyper-immune globulin (H-Ig) products from patients recovering from COVID-19 [46]. Plasma samples from participants of COVID vaccine trials will be advantageous for the production of hyper-immune immunoglobulins with greater specificity and high titers. Such a pharmaceutical product containing a cocktail of virus-specific antibodies will be easy to distribute among people. Another promising alternative for the production of antibodies is the immunization of horses with inactivated SARS-CoV-2 antigen. The disadvantage of using equine antibodies is that it might lead to adverse allergic reactions in human patients, probably due to activation of the human immune system. The stable version of recombinant viral proteins has been developed in different laboratories. These laboratory constructs proteins are injected into genetically modified mice for the production of virus-specific antibodies [47,48]. Moreover, genetic engineering has made it possible to create a more humanized form of monoclonal antibodies suitable. The possibility of isolating antibody-producing B cells to generate hybridomas and artificially synthesize antibodies using recombinant technology might serve better therapeutic options in clinical research [49-53].
Limitations of the Review
There are some important limitations of the present study that should be noted. This review was not intended to be a more comprehensive review regarding the utility of convalescent plasma therapy for the treatment of COVID-19. Rather, it was designed to address the possibility of convalescent plasma-derived hyper-immune immunoglobulins as a new therapeutic approach in the current situation. At the time of writing this review, however, limited information was available on the implementation of hyper-immune immunoglobulins for the treatment of COVID-19. It should also be noted that our review mainly focused on the studies carried out during the initial phase of the COVID-19 outbreak in Asian countries including China, Korea, Taiwan. As such, more rigorous research and additional data from other countries would be beneficial to understand the outcomes of convalescent plasma therapy or its derivatives as the treatment option for COVID-19.
Conclusion
Though several potential vaccines/antiviral drugs are in pipeline, getting an approved candidate for rational use will take some time. Under such circumstances, therapeutic antibodies can offer several advantages over conventional pharmacotherapy and, therefore, have implications for the treatment of several infectious diseases. Convalescent plasma-derived hyper-immune antibodies provide a ray of hope for control of the contagious disease such as the COVID-19, for which there is no vaccine. Nevertheless, several setbacks need to be dealt with before using hyperimmune globulins as a therapeutic strategy for the COVID-19 pandemic.
Declarations
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
There are no funding sources for this work.
References
- Kaufmann, Stefan HE. "Remembering Emil von Behring: From tetanus treatment to antibody cooperation with phagocytes." mBio, Vol. 8, No. 1, 2017, p. e00117.
- Hey, Adam. "History and practice: Antibodies in infectious diseases." Antibodies for Infectious Diseases, 2015, pp. 1-21.
- Graham, Barney S., and Donna M. Ambrosino. "History of passive antibody administration for prevention and treatment of infectious diseases." Current Opinion in HIV and AIDS, Vol. 10, No. 3, 2015, pp. 129-34.
- Pucca, Manuela B., et al. "History of envenoming therapy and current perspectives." Frontiers in Immunology, Vol. 10, 2019, p. 1598.
- Leon, Guillermo, et al. "Current technology for the industrial manufacture of snake antivenoms." Toxicon, Vol. 151, 2018, pp. 63-73.
- Maria Gutierrez, Jose, et al. "Antivenoms for snakebite envenomings." Inflammation and Allergy-Drug Targets (Formerly Current Drug Targets-Inflammation and Allergy) (Discontinued), Vol. 10, No. 5, 2011, pp. 369-80.
- Deka, Archana, et al. "Comparative analysis of Naja kaouthia venom from North-East India and Bangladesh and its cross reactivity with Indian polyvalent antivenoms." Toxicon, Vol. 164, 2019, pp. 31-43.
- Deka, Archana, et al. "Proteomics of Naja kaouthia venom from North East India and assessment of Indian polyvalent antivenom by third generation antivenomics." Journal of Proteomics, Vol. 207, 2019, p. 103463.
- Keller, Margaret A., and E. Richard Stiehm. "Passive immunity in prevention and treatment of infectious diseases." Clinical Microbiology Reviews, Vol. 13, No. 4, 2000, pp. 602-14.
- Garraud, O., et al. "Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow." Transfusion Clinique et Biologique, Vol. 23, No. 1, 2016, pp. 39-44.
- Marson, Piero, Andrea Cozza, and Giustina De Silvestro. "The true historical origin of convalescent plasma therapy." Transfusion and Apheresis Science, Vol. 59, No. 5, 2020, p. 102847.
- Cheng, Y., et al. "Use of convalescent plasma therapy in SARS patients in Hong Kong." European Journal of Clinical Microbiology and Infectious Diseases, Vol. 24, No. 1, 2005, pp. 44-46.
- Yeh, Kuo-Ming, et al. "Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital." Journal of Antimicrobial Chemotherapy, Vol. 56, No. 5, 2005, pp. 919-22.
- Hung, Ivan FN, et al. "Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection." Clinical Infectious Diseases, Vol. 52, No. 4, 2011, pp. 447-56.
- Zhou, Boping, Nanshan Zhong, and Yi Guan. "Treatment with convalescent plasma for influenza A (H5N1) infection." New England Journal of Medicine, Vol. 357, No. 14, 2007, pp. 1450-51.
- Ko, Jae-Hoon, et al. "Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: A single centre experience." Antiviral Therapy, Vol. 23, No. 7, 2018, pp. 617-22.
- Arabi, Yaseen M., et al. "Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia." Emerging Infectious Diseases, Vol. 22, No. 9, 2016, pp. 1554-61.
- Winkler, Anne M., and Scott A. Koepsell. "The use of convalescent plasma to treat emerging infectious diseases: Focus on Ebola virus disease." Current Opinion in Hematology, Vol. 22, No. 6, 2015, pp. 521-26.
- Fischer, Johannes C., et al. "The role of passive immunization in the age of SARS-CoV-2: An update." European Journal of Medical Research, Vol. 25, No. 1, 2020, pp. 1-6.
- Tiberghien, Pierre, et al. "Collecting and evaluating convalescent plasma for COVID-19 treatment: Why and how?" Vox Sanguinis, Vol. 115, No. 6, 2020, pp. 488-94.
- Gozalbo-Rovira, Roberto, et al. "SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients." Journal of Clinical Virology, Vol. 131, 2020, p. 104611.
- Zhang, Libo, et al. "Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19." Aging (Albany NY), Vol. 12, No. 8, 2020, pp. 6536-42.
- Seydoux, Emilie, et al. "Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation." Immunity, Vol. 53, No. 1, 2020, pp. 98-105.
- Bloch, Evan M., et al. "Guidance for the procurement of COVID-19 convalescent plasma: Differences between high-and low-middle-income countries." Vox Sanguinis, Vol. 116, No. 1, 2021, pp. 18-35.
- Shen, Chenguang, et al. "Treatment of 5 critically ill patients with COVID-19 with convalescent plasma." JAMA, Vol. 323, No. 16, 2020, pp. 1582-89.
- Chen, Binzhen, and Rong Xia. "Early experience with convalescent plasma as immunotherapy for COVID-19 in China: Knowns and unknowns." Vox Sanguinis, Vol. 115, No. 6, 2020, pp. 507-14.
- Ye, Mingxiang, et al. "Treatment with convalescent plasma for COVID-19 patients in Wuhan, China." Journal of Medical Virology, Vol. 92, No. 10, 2020, pp. 1890-901.
- Duan, Kai, et al. "Effectiveness of convalescent plasma therapy in severe COVID-19 patients." Proceedings of the National Academy of Sciences, Vol. 117, No. 17, 2020, pp. 9490-96.
- Li, Ling, et al. "Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial." JAMA, Vol. 324, No. 5, 2020, pp. 460-70.
- Chai, Khai Li, et al. "Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review." Cochrane Database of Systematic Reviews, Vol. 10, 2020.
- Nagoba, Basavraj, et al. "Positive aspects, negative aspects and limitations of plasma therapy with special reference to COVID-19." Journal of Infection and Public Health, Vol. 13, No. 12, 2020, pp. 1818-22.
- Gajic, Ognjen, et al. "Transfusion-related acute lung injury in the critically ill: Prospective nested case-control study." American Journal of Respiratory and Critical Care Medicine, Vol. 176, No. 9, 2007, pp. 886-91.
- Bloch, Evan M., et al. "Deployment of convalescent plasma for the prevention and treatment of COVID-19." The Journal of Clinical Investigation, Vol. 130, No. 6, 2020, pp. 2757-65.
- Zhao, Qian, and Yong He. "Challenges of convalescent plasma therapy on COVID-19." Journal of Clinical Virology, Vol. 127, 2020, p. 104358.
- MacLennan, Sheila, and John AJ Barbara. "Risks and side effects of therapy with plasma and plasma fractions." Best Practice and Research Clinical Haematology, Vol. 19, No. 1, 2006, pp. 169-89.
- World Health Organization. "Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks: interim guidance for national health authorities and blood transfusion services." No. WHO/HIS/SDS/2014.8. World Health Organization, 2014.
- Zhang, Bin, et al. "Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection." Chest, Vol. 158, No. 1, 2020, pp. e9-e13.
- Zhang, Zhan, et al. "Purification of severe acute respiratory syndrome hyperimmune globulins for intravenous injection from convalescent plasma." Transfusion, Vol. 45, No. 7, 2005, pp. 1160-64.
- Burnouf, Thierry, and Mirjana Radosevich. "Affinity chromatography in the industrial purification of plasma proteins for therapeutic use." Journal of Biochemical and Biophysical Methods, Vol. 49, No. 1-3, 2001, pp. 575-86.
- El-Ekiaby, Magdy, et al. "Minipool caprylic acid fractionation of plasma using disposable equipment: A practical method to enhance immunoglobulin supply in developing countries." PLoS Neglected Tropical Diseases, Vol. 9, No. 2, 2015, p. e0003501.
- Hemming, Val G. "Use of intravenous immunoglobulins for prophylaxis or treatment of infectious diseases." Clinical and Diagnostic Laboratory Immunology, Vol. 8, No. 5, 2001, pp. 859-63.
- Hsu, Jennifer L., and Nasia Safdar. "Polyclonal immunoglobulins and hyperimmune globulins in prevention and management of infectious diseases." Infectious Disease Clinics, Vol. 25, No. 4, 2011, pp. 773-88.
- Hung, Ivan FN, et al. "Hyperimmune IV immunoglobulin treatment: A multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A (H1N1) infection." Chest, Vol. 144, No. 2, 2013, pp. 464-73.
- Chi, Xiangyang, et al. "A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2." Science, Vol. 369, No. 6504, 2020, pp. 650-55.
- Zhou, Daming, et al. "Structural basis for the neutralization of SARS-CoV-2 by an antibody from a convalescent patient." Nature Structural and Molecular Biology, Vol. 27, No. 10, 2020, pp. 950-58.
- Efantis, Amy Chevalier, Brenna Raines, and Larisa Cervenakova. "Industry call for COVID-19 convalescent plasma collections." ISBT Science Series, Vol. 15, No. 4, 2020, pp. 415-16.
- Chen, Wen-Hsiang, Peter J. Hotez, and Maria Elena Bottazzi. "Potential for developing a SARS-CoV Receptor-Binding Domain (RBD) recombinant protein as a heterologous human vaccine against coronavirus infectious disease (COVID)-19." Human Vaccines and Immunotherapeutics, Vol. 16, No. 6, 2020, pp. 1239-42.
- He, Yuxian, et al. "Antigenic and immunogenic characterization of recombinant baculovirus-expressed severe acute respiratory syndrome coronavirus spike protein: Implication for vaccine design." Journal of Virology, Vol. 80, No. 12, 2006, pp. 5757-67.
- Köhler, Georges, and Cesar Milstein. "Continuous cultures of fused cells secreting antibody of predefined specificity." Nature, Vol. 256, No. 5517, 1975, pp. 495-97.
- Klasse, P. J., and John P. Moore. "Antibodies to SARS-CoV-2 and their potential for therapeutic passive immunization." Elife, Vol. 9, 2020, p. e57877.
- Zhou, Guangyu, and Qi Zhao. "Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2." International Journal of Biological Sciences, Vol. 16, No. 10, 2020, pp. 1718-23.
- Shanmugaraj, Balamurugan, et al. "Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19)." Asian Pacific Journal of Allergy and Immunology, Vol. 38, No. 1, 2020, pp. 10-18.
- Ju, Bin, et al. "Human neutralizing antibodies elicited by SARS-CoV-2 infection." Nature, Vol. 584, No. 7819, 2020, pp. 115-19.