Research - International Journal of Medical Research & Health Sciences ( 2022) Volume 11, Issue 3
Histopathological Grades of Oral Squamous Cell Carcinoma a Prognostic Indicator: Hospital-Based Study
Sabiha Mokashi Khan1*, Nilima Prakash2, Rizwan Raiskhan Mokashi3, Sajda Khan Gajdhar4, Vaishali Sadhwani2 and Mohnish Zulfikar Manva52Department of Oral Pathology and Microbiology, MGV’s KBH Dental College and Hospital, Nashik, Maharashtra, India
3Department of Conservative Dentistry and Endodontics, SMBT IDSR Dental College Dhamangaon, Nashik, Maharashtra, India
4Department of Oral Pathology and Microbiology, Oral Basic and Clinical Science, Ibn Sina National College of Medical Studies, Jeddah, KSA
5Department of Restorative Dental Science, College of Dentistry, Majmaah University, KSA
Sabiha Mokashi Khan, Oral Cancer Screening and Awareness in Cancer Screening Vertical, Indian Cancer Society, Mumbai, India, Email: dr.sabihamokashi@gmail.com
Received: 01-Mar-2022, Manuscript No. ijmrhs-22-55846 (M); Editor assigned: 03-Mar-2022, Pre QC No. ijmrhs-22-55846 (P); Reviewed: 04-Mar-2022, QC No. ijmrhs-22-55846 (Q); Revised: 12-Mar-2022, Manuscript No. ijmrhs-22-55846 (R); Published: 20-Mar-2022
Abstract
Background: Oral Squamous Cell Carcinoma (OSCC) is a major public health problem with an increasing incidence and mortality rate, primarily because of the prevalent oral habits of betel quid chewing, smoking, alcohol consumption, and poor quality of life. Despite advances in diagnostic and therapeutic intervention the 5-year survival rate of patients with OSCC has not improved. Oral squamous cell carcinoma has a great proneness to produce metastasis in lymph nodes and further reduces the survival rate. Histopathological grades of OSCC can serve as a strong prognostic indicator. Objective: 1. To study the prevalence of oral squamous cell carcinoma about patient demographic parameters (age, sex, and site of lesion). 2. Analyse the relation between histopathological grades of OSCC and regional lymph node metastasis. Material and Method: A total of 30 cases of histopathological diagnosed OSCC. Sections stained with hematoxylin and eosin were graded according to Bryne’s grading system. Out of 30 cases of OSCC, 13 were Well-differentiated, 12 moderately differentiated, and 5 poorly differentiated cases of OSCC. Statistical analysis was executed by using the SPSS version 20 software and Chi-Square analysis was performed. Result: The statistical analysis of the correlation between the different grades of OSCC with patient’s gender and age and site of involvement was found to be non-significant. A significant relation was evident between histological grades of OSCC and cervical lymph node metastasis. (p=0.0010*) Conclusion: In our study, it can be concluded that OSCC is more common in males in their 5th to 7th decade of their life with the most common site being buccal mucosa and tongue. The majority of cases are well-differentiated followed by moderate and poorly differentiated OSCC. In our study, a significant relation was evident between histological grades and cervical lymph node metastasis suggesting that higher grades of OSCC have a higher risk of the metastatic lymph node.
Keywords
Oral Squamous Cell Carcinoma (OSCC), Metastasis, Cervical lymph nodes, Histological grades of OSCC, Prognostic indicator
Introduction
Oral cancer is one of the most common malignancies worldwide, with an estimated 53,260 new cases and 10,750 deaths in 2020. Oral Squamous Cell Carcinoma (OSCC) accounts for almost 90% of all malignant tumors of the oral cavity [1].
It most commonly affects men in the 6th to 8th decades of life and is rare in patients younger than 40 years [2]. It more frequently affects males than females (M: F=1.5:1) mainly because more men than women indulge in high-risk habits [3]. Squamous cell carcinoma affects all areas of the oral cavity, but the most commonly reported sites are the tongue, floor of the mouth, and lower lip [2].
The patients diagnosed with early-stage OSCC have substantially improved prognoses. But the overall survival rate in patients with advanced OSCC has not improved significantly during the past four decades. New procedures such as Gene expression profiling for early detection, risk assessment, and early intervention are ongoing to improve the survival rate in patients with OSCC [4]. Therefore, studies construing the oncological behavior of OSCC are needed [1].
The main criteria routinely used for prognostication and treatment of OSCC are clinical staging based on TNM classification and the location of the tumor. However, variations in the treatment response and prognosis are quite high for OSCCs, with some patients presenting tumors at the same site and same clinical stage having prolonged survival, while others may die due to rapid metastasis. Given those difficulties, it has been proposed that a detailed histopathological grading of OSCC with specific histopathological scoring systems could help in the individualization of treatment and prognostication of patients with OSCC [5].
TNM classification has been widely used to plan treatment, estimate prognosis, and evaluate treatment results. The 8th edition of the AJCC staging manual has added Depth of Invasion (DOI) and Extra Nodal Extension (ENE) as modifiers to the T and N category of OCC staging, respectively [6]. Histologic grade is not included in this current staging criteria. Historically, in head and neck Squamous Cell Carcinoma (SCC), the histologic grade has not been considered as a prognostic factor. Though, in many other solid tumors, the histological grade is known to affect prognosis [7].
However, few key studies of grades are informative. Brandwein-Gensler, et al., found histologic risk assessment to be a better predictor than margin status for predicting local recurrence and overall survival [8]. Piffko, et al., reported that their histopathological malignancy grade of the invasive front (tumour front score) proved to be the more powerful prognostic indicator than tumor size, nodal status, or margin status [9].
Metastatic cervical lymphadenopathy is the most reliable prognostic indicator of treatment outcomes in patients with oral squamous cell carcinoma. The survival rate of the patient decreases by 50% with the presence of regional lymphadenopathy [10].
Hence, the histologic grade should be considered clinically when making treatment decisions and should include being included as a covariate to improve prognostic accuracy. Our study aims to determine the prevalence of oral squamous cell carcinoma about patient demographic parameters (age, sex, and site of lesion) and to analyze the relation between histopathological grades of OSCC and regional lymph node metastasis.
Materials and Methods
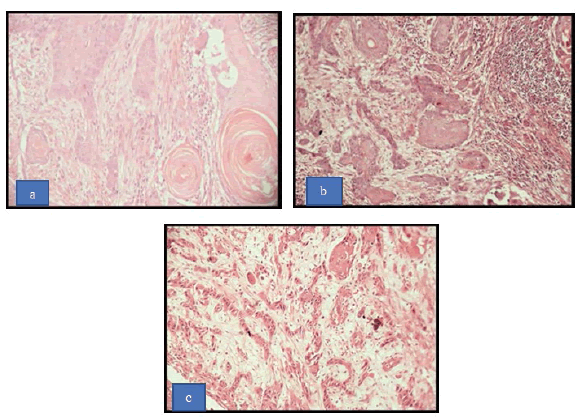
The study was conducted at the institute hospital. The study was approved by the Institutional Ethical Committee informed consent was obtained from patients for the present study. This retrospective study comprised 30 histopathological diagnosed cases of Oral Squamous Cell Carcinoma (OSCC) in a year from 2019-to 2020. Relevant information of the patient (age, sex, site of the lesion, habit history, duration, and frequency of habit) was recorded. The staging of OSCC was done according to the staging system proposed by the American Joint Committee for Cancer Staging and End Results Reporting (AJCCS) known as the TNM system [11]. The paraffin block was inspected to confirm the adequate size of the tissue with the most representing area. The section was stained with Haematoxylin and Eosin and a detailed histopathological examination was done for each of these lesions under a light microscope. The cases were graded as well-differentiated, moderately differentiated, and poorly differentiated OSCC according to Bryne’s grading system (Figure 1) [12].
Statistical Analysis
All the parameters of each patient according to the clinical and histopathologic reports were collated in the datasheet. Statistical analysis was executed by using the SPSS version 20 software. Chi-Square analysis was done for clinicopathological correlation of different grades of oral squamous cell carcinoma with patient’s age, sex, and site of the lesion, correlation histopathological grades of OSCC, and lymph node metastasis.
Result
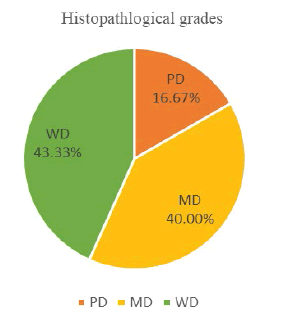
The present study comprised of 30 cases, 13 (43.33%) cases of OSSC were categorized as Grade I (Well differentiated), 12 (40.00%) cases fell under the category of Grade II (Moderately differentiated) and 5 (16.67%) cases were Grade III (Poorly differentiated) according to the Bryne’s grading system (Table 1 and Figure 2).
| Histopathological grades | Number of cases | Percentage (%) |
|---|---|---|
| WD | 13 | 43.33 |
| MD | 12 | 40.00 |
| PD | 5 | 16.67 |
| Total | 30 | 100.00 |
| WD: Well Differentiated, MD: Moderately Differentiated; PD: Poorly Differentiated | ||
Among these cases 25 (83.33%) patients are male and 5 (16.67%) patients are female (Table 2). Chi-square analysis of the gender distribution when comparing with different grades of OSCC was found to be statistically non-significant (p=0.5000).
Among 30 cases, 11 (36.67) patients were in the age group of 30-50 years and 19 (63.33%) patients were in the age group of 51-70 years in comparison with different grades of OSCC as shown in Table 2. Chi-square analysis of the age-wise distribution with different grades of OSCC was found to be statistically non-significant. (p=0.2280).
| Parameters | Histopathological grades of OSCC | p-value | ||
|---|---|---|---|---|
| Well-differentiated | Moderately differentiated | Poorly differentiated | ||
| Age group | ||||
| 30-50 | 7 (63.64%) | 3 (27.27%) | 1 (9.09%) | 0.2280 |
| 51-70 | 6 (31.58%) | 9 (47.37%) | 4 (21.05%) | |
| Gender | ||||
| Male | 10 (40.00%) | 10 (40.00%) | 5 (20.00%) | 0.5000 |
| Female | 3 (60.00%) | 2 (40.00%) | 0 (0.00%) | |
| Site | ||||
| Floor of the mouth | 1 (33.33%) | 1 (33.33%) | 1 (33.33%) | 0.4800 |
| Buccal mucosa | 10 (50.00%) | 7 (35.00%) | 3 (15.00%) | |
| Tongue | 2 (40.00%) | 2 (40.00%) | 1 (20.00%) | |
| Alveolus | 0 (0.00%) | 2 (100.00%) | 0 (0.00%) | |
In this study, the most common site of involvement is buccal mucosa 20 (66.67%). The second most common site of involvement is tongue 5 (16.67%) cases. Chi-square analysis of the site-wise distribution with different grades of OSCC was found to be statistically non-significant (p=0.4800) Table 2.
On comparing the histological grades of OSCC with regional lymph nodes, 12 (63.16%) of well-differentiated OSCC cases were N0, 7 (36.84%) of moderately differentiated OSCC cases were N0. 1 (9.09%) of well-differentiated showed N1, 5 (45.45%) of moderately differentiated OSCC showed N1 and 5 (16.67%) of poorly differentiated OSCC cases showed N1. On Comparison with different grades of OSCC and regional lymph node metastasis, as shown in Table 3, Chi-square analysis of the was found to be statistically significant. (p=0.0010*).
| Regional lymph node | Histopathological grades | |||||||
|---|---|---|---|---|---|---|---|---|
| WD | % | MD | % | PD | % | Total | % | |
| N0 | 12 | 63.16 | 7 | 36.84 | 0 | 0.00 | 19 | 63.33 |
| N1 | 1 | 9.09 | 5 | 45.45 | 5 | 45.45 | 11 | 36.67 |
| Total | 13 | 43.33 | 12 | 40.00 | 5 | 16.67 | 30 | 100.00 |
| p=0.0010* | ||||||||
Discussion
Oral cancer is the commonest form of cancer in are head and neck. OSCC prevalence is different in various parts of the world [13]. In the present study majority of the patients were in the ages group of (51-70) yrs (63.33%) among which 47.37% were moderately differentiated, followed by well-differentiated 31.58% and 21.05% were poorly differentiated. Previous studies have suggested WDSCC as the most common type of OSCC which is followed by MDSCC and PDSCC [14]. There is a long-standing debate about the prognosis and aggression of Oral SCC in young patients as compared to older patients and several studies have proposed the need for additional research on this topic [15].
The majority of our patients were male 83.33%, this gender disparity is due to cultural trends in our country where males can practice the habit of tobacco chewing or smoking whereas females refrain from adopting such habits due to social and traditional stigma.
In our study, the most common site of involvement is buccal mucosa 66.67%. The habit of tobacco chewing frequently with keeping tobacco quid against the buccal mucosa at the vestibule. The next frequent site after buccal mucosa was the tongue 16.67%. The majority of the cases were well-differentiated OSCC followed by moderate and poorly differentiated.
The statistical analysis of this correlative study between the histological grades of OSCC and patient’s gender and age and site of involvement was found to be non-significant.
Abdul Shaikh, et al., correlated histopathological patterns of OSCC with age and site, with a statistically significant value between these two correlations [16].
On the correlation of histopathological grades of OSCC with regional lymph node metastasis 19 (63.33%), cases showed no regional lymph node metastasis. Whereas 11 (36.67%) showed lymph node metastasis of which 5 (45.45%) cases were moderately differentiated squamous cell carcinoma and 5 (45.45%) poorly differentiated squamous cell carcinoma with the statistically significant result (p=0.0010*). Similar results were obtained by Y Okada in his study, suggesting that histological malignancy can serve as a predictor for metastasis in the cervical lymph nodes [17]. A similar result was obtained by Sumana C, et al., in their study higher incidence of lymph node metastasis was noted in moderately and poorly differentiated SCC of the oral tongue [10].
Nodal metastasis transpires when tumor cells from the primary site penetrate the lymphatic channels and migrate to regional lymph nodes in the neck. The most common site for OSCC metastasis in cervical lymph nodes, which reduces the survival rate by 50%. Histologically, tumor cells dissemination outside the lymph node capsule makes the prognosis worse and reduces patient survival rate [13].
Progression of the disease is the single most important aspect of malignant neoplasms, which is complex and needs correct assessment of the overall survival of a patient, which resulted in frantic search of important histological indicators which could benefit the current clinical staging system and find a panel of strong independent predictors of prognosis or survival rate in patients with Oral Squamous Cell Carcinoma (OSCC) [18].
The limitation of our study is the small sample size and its uni-centric nature, which may be the reasons for differences between our results and previous studies. Multi-centric studies on histological grades of OSCC and its prognostic value are needed.
Conclusion
Early detection of malignancy is very important to improve the prognosis and the patient survival rate. Unfortunately, most of the cases are identified in the advanced stage. In our study, from a demographic point of view, it can be concluded that OSCC is more common in males in their 5th to 7th decade of their life with the most common site being buccal mucosa and tongue. The majority of cases are well-differentiated followed by moderate and poorly differentiated OSCC. In our study, a significant result was obtained between histological grades and cervical lymph node metastasis suggesting that higher grades of OSCC have a higher risk of lymph node metastasis. Hence, the therapeutic regimen should be based not only on TNM classification but also on the evaluation of histological grades of OSCC in the future.
Declarations
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Tran, Cuong Minh, et al. "Clinicopathological and immunohistochemical characteristics of pigmented oral squamous cell carcinoma." Oncology Letters, Vol. 21, No. 4, 2021, pp. 1-9.
Google Scholar Crossref - Rai, Harish Chandra, and Junaid Ahmed. "Clinicopathological correlation study of oral squamous cell carcinoma in a local Indian population." Asian Pacific Journal of Cancer Prevention, Vol. 17, No. 3, 2016, pp. 1251-54.
Google Scholar Crossref - Feller, Liviu, and Johan Lemmer. "Oral squamous cell carcinoma: Epidemiology, clinical presentation and treatment." Journal of Cancer Therapy, Vol. 3, No. 4, 2012.
Google Scholar Crossref - Chen, Chu, et al. "Gene expression profiling identifies genes predictive of oral squamous cell carcinoma." Cancer Epidemiology and Prevention Biomarkers, Vol. 17, No. 8, 2008, pp. 2152-62.
Google Scholar Crossref - Sawazaki-Calone, I., et al. "The prognostic value of histopathological grading systems in oral squamous cell carcinomas." Oral Diseases, Vol. 21, No. 6, 2015, pp. 755-61.
Google Scholar Crossref - Zanoni, Daniella Karassawa, and Snehal G. Patel. "New AJCC: How does it impact oral cancers?" Oral Oncology, Vol. 104, 2020, p. 104607.
Google Scholar Crossref - Thomas, Brian, Margaret Stedman, and Louise Davies. "Grade as a prognostic factor in oral squamous cell carcinoma: A populationâ?ÂÂbased analysis of the data." The Laryngoscope, Vol. 124, No. 3, 2014, pp. 688-94.
Google Scholar Crossref - Brandwein-Gensler, Margaret, et al. "Oral squamous cell carcinoma: Histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival." The American Journal of Surgical Pathology, Vol. 29, No. 2, 2005, pp. 167-78.
Google Scholar Crossref - Piffko, J., et al. "Prognostic value of histobiological factors (malignancy grading and AgNOR content) assessed at the invasive tumour front of oral squamous cell carcinomas." British Journal of Cancer, Vol. 75, No. 10, 1997, pp. 1543-46.
Google Scholar Crossref - Viswanatha, Sumana C., Naveen Hedne, and Suhel Hasan. "Correlation between histological grading, LVI and PNI of carcinoma oral tongue to lymph node metastasis." International Journal of Otorhinolaryngol Head and Neck Surgery, Vol. 5, No. 1, 2019, pp. 159-64.
Google Scholar Crossref - Kim, Kyubo, and Dong Jin Lee. "The updated AJCC/TNM staging system for oral tongue cancer." Translational Cancer Research, Vol. 8, No. Suppl 2, 2019, pp. S164-66.
Google Scholar Crossref - Bhargava, Ankur, Sonal Saigal, and Monali Chalishazar. "Histopathological grading systems in oral squamous cell carcinoma: A review." Journal of International Oral Health, Vol. 2, No. 4, 2010, pp. 1-10.
Google Scholar Crossref - Bugshan, Amr, and Imran Farooq. "Oral squamous cell carcinoma: Metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis." F1000Research, Vol. 9, 2020, p. 229.
Google Scholar Crossref - Rahaman, Syed Mukith Ur, and BR Ahmed Mujib. "Histopathological correlation of oral squamous cell carcinoma among younger and older patients." Journal of Oral and Maxillofacial Pathology: JOMFP, Vol. 18, No. 2, 2014, pp. 183-88.
Google Scholar Crossref - Falaki, Farnaz, et al. "Clinical and histopathological analysis of oral squamous cell carcinoma of young patients in Mashhad, Iran: A retrospective study and review of literatures." Oral Medicine, Oral Pathology and Oral Surgery, Vol. 16, No. 4, 2011, pp. e473-77.
Google Scholar Crossref - Shaikh, Abdul Hafeez, Taqi Muhammad, and Tazeen Rasheed. "Evaluating the correlation between histopathological patterns of oral squamous cell carcinoma, age and site." Pakistan Oral & Dental Journal, Vol. 35, No. 1, 2015.
Google Scholar Crossref - Okada, Y., et al. "An analysis of cervical lymph nodes metastasis in oral squamous cell carcinoma: Relationship between grade of histopathological malignancy and lymph nodes metastasis." International Journal of Oral and Maxillofacial Surgery, Vol. 32, No. 3, 2003, pp. 284-88.
Google Scholar Crossref - Sethi, Sneha, et al. "Histopathological factors in oral squamous cell carcinoma-Should a clinician look beyond clinical staging?" Journal of Oral and Maxillofacial Surgery, Vol. 79, No. 8, 2021, pp. 1694-705.
Google Scholar Crossref