Review - International Journal of Medical Research & Health Sciences ( 2022) Volume 11, Issue 10
Laboratory Based Mortality Prediction in Covid-19 patients
Sherif Sabry*, Kerollos N. Naguib, Mohamed A. Hamila and Ahmed YassinSherif Sabry, Department of Critical Care Medicine, Beni Suef University, Egypt, Email: shsabri2014@gmail.com
Received: 13-Sep-2022, Manuscript No. ijmrhs-22-74546; Editor assigned: 15-Sep-2022, Pre QC No. ijmrhs-22-74546 (PQ); Reviewed: 04-Oct-2022, QC No. ijmrhs-22-74546 (Q); Revised: 24-Oct-2022, Manuscript No. ijmrhs-22-74546 (R); Published: 30-Oct-2022
Abstract
Background: Several markers were linked to adverse clinical outcomes in critically ill patients with COVID-19. Previous reports showed that elevated Neutrophil-to-Lymphocyte Ratio (NLR), C-Reactive Protein (CRP), D-dimer, vitamin D levels, and serum ferritin are associated with worse outcomes and increased mortality of critically ill patients with COVID-19; however, evidence is still insufficient to implement the predictive values of these markers in the management algorithm for the patients. Objectives: To evaluate the predictive value of five blood markers on the outcomes of critically ill COVID-19 patients. Methods: We performed a prospective study that included 40 critically ill patients with COVID-19 (COVID severity index ≥ 8) who were admitted to the Intensive Care Unit (ICU) of a tertiary hospital in Egypt. Blood samples for the studied markers were collected within two days of admission. Results: Forty patients were included, with a mean age of 55.6 ± 9.9 years and equal gender distribution. Nearly 62.5% of the patients needed mechanical ventilation, with mean days of ventilation of 15 days. The SOFA score after 48 hours was 9 ± 2.8. Fifty-five percent of participants needed high doses of vasopressors. The mean length of stay in the ICU was 17.7 days ± 5.5 days, and the mortality occurred in 55% of participants. There was a trend towards an association between mortality and male sex, presence of diabetes mellites, bilateral infiltration of lungs, and heart failure. After admission, the serum CRP, ferritin, D-dimer, NLR, and SOFA score after 48 hours had a significant role in the prediction of mortality. Both NLR and D-dimer had the highest area under the curve and sensitivity (95.5% for NLR vs. 90.9% for D-dimer), specificity (100% for both), PPV, and NPV at a cut-off value of 5.5 and 0.85 for both, respectively. Besides, the vitamin D level after 48 hours had a significant role in predicting mortality at a cut-off ≤ 18. Conclusions: CRP, NLR, and D-dimer were found to be reliable predictors of COVID-19 outcomes, including critical illness and mortality. Elevated serum ferritin and vitamin D an be used as supplementary predictors but cannot be relied on as independent predictors. The interpretation of these biomarkers should be correlated with many demographic and clinical factors
Keywords
COVID-19, Mechanical ventilation, Mortality, ICU
Introduction
The Coronavirus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARSCoV-2) was first reported in the city of Wuhan (China) in December 2019 and was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020 [12]. During the last several months, COVID-19 has posed major risks to global health by placing unprecedented strain on healthcare systems around the world. Creactive protein and many pro-inflammatory cytokines and chemokines, such as interferon-gamma (IFN-γ), interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) are all considerably elevated in the blood of patients with severe COVID-19 symptoms [3-5]. When these cytokines are produced in large quantities, they cause a systemic inflammatory response that boosts CD8+ cytotoxic T-cell activity while simultaneously decreasing regulatory T-cell activity [6]. As a result of these abnormal immune responses, patients may develop multiorgan failure and acute respiratory distress syndrome [7,8]. In severe cases of COVID-19, there has been observed an increase in neutrophil counts and a substantial drop in peripheral lymphocyte counts (mainly CD4+ and CD8+ T cells), with the degree of lymphopenia corresponding with disease severity [9]. Abnormal coagulation outcomes are also seen in severe cases of COVID-19. These include significantly higher amounts of Ddimer, fibrinogen, and other fibrin degradation products, substantially prolonged activated partial thromboplastin time, and prothrombin time [9,10]. These findings raise the possibility of the emergence of overt disseminated intravascular coagulation [10]. Mechanistic studies have indicated that vitamin D has anti-inflammatory and immunomodulatory effects, in addition to its well-known function in regulating calcium and bone homeostasis [11]. Vitamin D has been found to have a crucial role in the regulation of both innate and adaptive immune responses, with studies showing that it promotes tolerogenic responses, suppresses the production of various pro-inflammatory cytokines, and enhances antiviral effector mechanisms [12-15]. More than 1 billion people of all ages are affected by vitamin D deficiency, making it a worldwide pandemic [16]. Furthermore, risk factors for vitamin D deficiency correlate with those with severe COVID-19 (such as obesity, older age, and Asian or Black ethnic origin) [17]. Vitamin D deficiency has been proposed by many studies as an independent risk factor for COVID-19 infection and detrimental consequences in the setting of preexisting illness [18]. This study aimed to evaluate the predictive value of five blood markers on the outcomes of critically ill COVID-19 patients.
Methods
The study gained ethical clearance from the responsible committee in the Faculty of Medicine, Beni-Suef University. Guardians of eligible patients were required to sign informed consent before deeming them eligible for the present study. The study was supported by a personal grant.
We performed a prospective study that included 40 critically ill COVID-19 (COVID severity index >8) patients admitted to the Critical Care Department of Beni-Suef University Hospital from January 2022 to March 2022. The decision of ICU admission was based solely on the treating physician's discretion without intervention from the study's investigators. Patients were deemed eligible if they had real-time reverse transcriptase-polymerase chain reaction (RT-PCR)-confirmed COVID-19, severe COVID-19 illness according to the National Early Warning Score-2 (NEWS-2) [19]. We excluded the pediatric age group, patients with tuberculosis, tumors, or pregnancy.
Each patient was evaluated for history and clinical examination findings, laboratory findings, sequential organ failure assessment score (SOFA score), Acute Physiology and Chronic Health Evaluation II (APACHE II), and chest Computed Tomography (CT) findings for COVID-19 Reporting and Data System (CO-RADS) classification [20,21].
Blood samples were withdrawn from the patients within 48 hours of admission. The laboratory investigations included a complete count with differential, CRP, D-dimer, serum ferritin, and vitamin D levels. Besides, routine laboratory investigations were conducted for the patients, including hepatic and renal functions, electrolytes, and arterial blood gas.
Study's Outcomes
We primarily assessed the association between the studied markers and in-hospital mortality. The secondary outcomes included the association between the studied markers and length of hospital stay, need for mechanical ventilation, duration of mechanical ventilation, and SOFA score.
Statistical Analysis
Retrieved data were summarized and processed with IBM SPSS statistical software (version 25). Frequencies were used to describe categories, and numeric were summarized into median (range). The hypothesis of significant associations between various parameters and clinical outcomes was tested by the Chi-square test for categorical variables and the Mann-Whitney test for continuous variables. The prediction utilities of the five markers were explored by receiver operator characteristics, and the outputs were presented with diagnostic accuracy measures. pvalue <0.05 was regarded as statistically significant.
Results
Forty patients were included, with a mean age of 55.6 ± 9.9 years and equal gender distribution. Nearly 62.5% of the patients needed mechanical ventilation, with mean days of ventilation of 15 days. The SOFA score after 48 hours was 9 ± 2.8. Fifty-five percent of participants needed high doses of vasopressors. The mean length of stay in the ICU was 17.7 ± 5.5 days, and mortality occurred in 55% of participants. Table 1 shows a statistically significant association between in-hospital mortality and increased age, high temperature, and higher APACHE II score. There was a trend towards an association between mortality and male sex, presence of diabetes mellites, bilateral infiltration of lungs, and heart failure.
Characteristics |
Alive (no=18) | Died (no=22) | p-value |
|---|---|---|---|
Age (mean ± SD) |
51.8 ± 10.8 | 58.7 ± 7.9 | 0.025* |
| Sex | |||
| Females | 10(50.0%) | 10(50.0%) | 0.525 |
| Males | 8(40.0%) | 12(60.0%) | |
| Diabetes with EOF | |||
| No | 10(58.8%) | 7(41.2%) | 0.131 |
| Yes | 8(34.8%) | 15(65.2%) | |
| HTN | |||
| No | 5(38.5%) | 8(61.5%) | 0.564 |
| Yes | 13(48.1%) | 14(51.9%) | |
| Heart failure | |||
| No | 8(53.3%) | 7(46.7%) | 0.412 |
| Yes | 10(40.0%) | 15(60.0%) | |
| COPD | |||
| No | 8(44.4%) | 10(55.6%) | 0.949 |
| Yes | 10(45.5%) | 12(54.5%) |
|
| Vital signs | |||
| Temperature | 37.9 ± 0.6 | 38.4 ± 0.3 | 0.001* |
| HR | 113.6 ± 8.4 | 117.4 ± 12.1 | 0.264 |
| RR | 29.8 ± 6 | 30.1 ± 3.2 | 0.768 |
| Systole | |||
| ≥ 90 mmHg | 12(46.2%) | 14(53.8%) | 0.842 |
| <90 mmHg | 6(42.9%) | 8(57.1%) | |
| SO2 | 84.4 ± 6 | 86.7 ± 4.6 | 0.179 |
| APACH II score | 12.8 ± 6.9 | 17.7 ± 6.1 | 0.023* |
| Bilateral infiltration of the lung | |||
| No | 7(70.0%) | 3(30.0%) | 0.067 |
| Yes | 11(36.7%) | 19(63.3%) | |
Table 2 shows that there was a statistically significant association between in-hospital mortality and high baseline levels of sodium, INR, and NLR among non-survivors. When the assessment was repeated after 48 hours, there was a significantly higher level of Total Leucocytic Count (TLC), CRP, serum ferritin, D-dimer, vitamin D, and NLR among non-survivors.
| Characteristics | Baseline | 48 hours | ||||
|---|---|---|---|---|---|---|
| Alive (no=18) | Died (no=22) | p-value | Alive (no=18) | Died | p-value | |
| (no=22) | ||||||
| Na | 131.6 ± 7.7 | 137.1 ± 8.8 | 0.040 | 135.8 ± 4.2 | 137.8 ± 4.6 | 0.166 |
| K+ | 3.9 ± 0.7 | 3.9 ± 0.8 | 0.818 | 3.7 ± 0.5 | 3.7 ± 0.5 | 0.963 |
| Ca+ | 7.7 ± 0.4 | 7.6 ± 0.5 | 0.492 | 8.5 ± 0.4 | 8.1 ± 0.7 | 0.059 |
| Create., | 1.5 ± 0.8 | 2.1 ± 1.4 | 0.135 | 1.5 ± 0.7 | 2.2 ± 1.2 | 0.036 |
| PO4 | 2.1 ± 0.3 | 2.2 ± 0.4 | 0.381 | 2.1 ± 0.3 | 2.1 ± 0.3 | 0.798 |
| Mg+ | 1.9 ± 0.2 | 1.9 ± 0.3 | 0.535 | 2.1 ± 0.3 | 2.1 ± 0.3 | 0.667 |
| Hb | 9.6 ± 1.5 | 9.9 ± 1.7 | 0.615 | 9.5 ± 1.5 | 9.9 ± 1.6 | 0.357 |
| TLC×103 | 11.6 ± 6.2 | 14.6 ± 6.2 | 0.138 | 9.9 ± 3.4 | 12.9 ± 3.5 | 0.009 |
| PLT×103 | 238.1 ± 122.1 | 219.1 ± 86.8 | 0.569 | 247.3 ± 114.9 | 223.1 ± 92.9 | 0.467 |
| INR | 1.3 ± 0.2 | 1.4 ± 0.3 | 0.524 | 1.4 ± 0.1 | 1.5 ± 0.2 | 0.192 |
| CRP | 54.6 ± 53.6 | 93.4 ± 69.7 | 0.06 | 54.9 ± 50.9 | 111.7 ± 72.15947 | 0.008 |
| Ferritin | 530.6 ± 259.1 | 649.9 ± 165.4 | 0.085 | 481.3 ± 278.4 | 735.7 ± 159.7 | 0.001 |
| D-dimer | 0.6 ± 0.1 | 0.8 ± 0.2 | 0.006 | 0.3 ± 0.1 | 1.3± 0.3 | 0.001 |
| Vit D | 21.6 ± 7.6 | 18.8 ± 2.4 | 0.114 | 20.2 ± 5.4 | 16.6 ± 2.3 | 0.007 |
| NLR | 2.4 ± 0.7 | 5.5 ± 1.3 | <0.001 | 1.7 ± 0.8 | 8 ± 1.3 | 0.001 |
| AST | 38.4 ± 25.9 | 53.7 ± 47.2 | 0.224 | 40.7 ± 18.6 | 55.6 ± 44.3 | 0.192 |
| ALT | 30.6 ± 25.5 | 43.2 ± 33.6 | 0.195 | 42.3 ± 19.2 | 48 ± 33.7 | 0.523 |
| Bilirubin | 1.1 ± 0.2 | 1.3 ± 0.4 | 0.061 | 1.1 ± 0.2 | 1.3 ± 0.4 | 0.137 |
| Albumin | 3.1 ± 0.3 | 2.9 ± 0.5 | 0.341 | 3.1 ± 0.3 | 2.9 ± 0.4 | 0.137 |
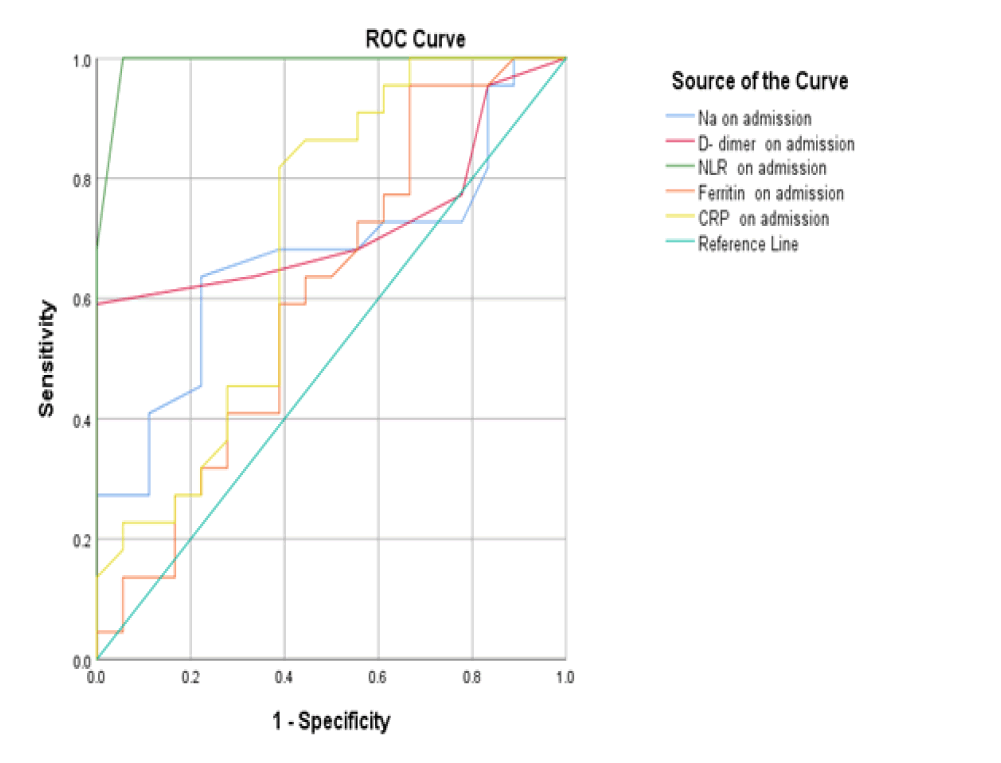
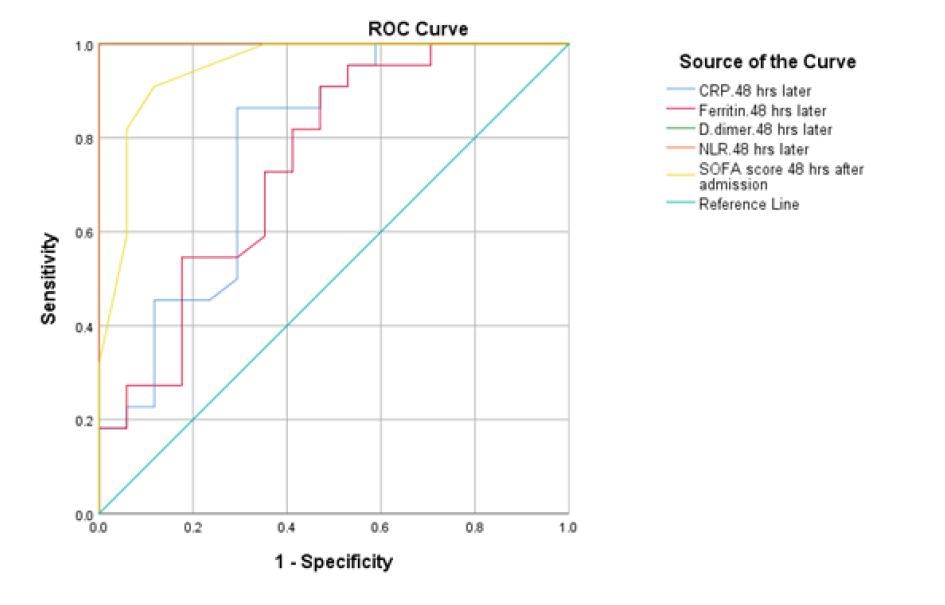
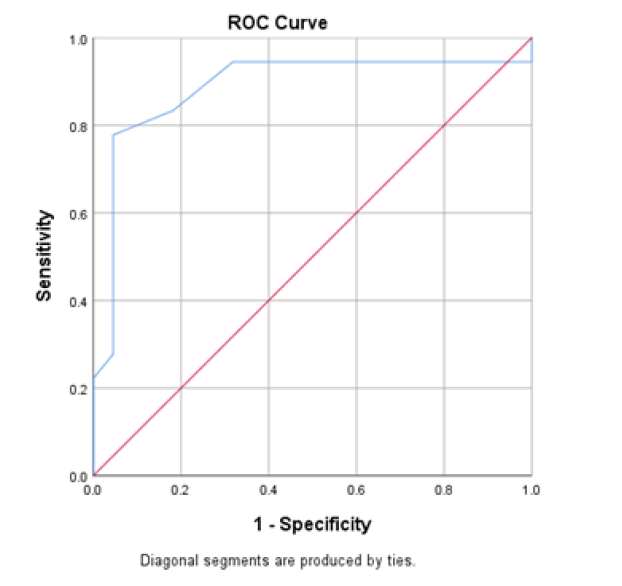
At baseline, serum D-dimer, NLR, and CRP on admission had a significant role in mortality prediction. Still, the highest one in prediction was NLR, with the highest under the curve and sensitivity (100%), specificity (94.6%), PPV, and NPV at a cut-off ≥ 3.5 (Table 3 and Figure 1). Likewise, 48 hours from admission, the serum CRP, ferritin, D-dimer, NLR, and SOFA score after 48 hours had a significant role in the prediction of mortality. Both NLR and Ddimer had the highest area under the curve and sensitivity (95.5 % for NLR vs. 90.9% for D-dimer), specificity (100% for both), PPV, and NPV at a cut-off value of 5.5 and 0.85 for both, respectively (Table 3 and Figure 2). Besides, the vitamin D level after 48 hours had a significant role in predicting mortality at a cut-off ≤ 18 (Figure 3).
| Variables | Area under curve | P-value | cut off | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) |
|---|---|---|---|---|---|---|---|
| On admission | |||||||
| D- dimer | 0.723 | 0.016 | ≥ 0.65 | 63.6 (50%-70%) | 66.7 (59%-78%) | 62 (55%-67%) | 65 (60%-75%) |
| Ferritin | 0.609 | 0.242 | ≥ 608 | 63.6 (55%-70%) | 55.6 (36%-61%) | 60 (47%-65%) | 52 (39%-55%) |
| CRP | 0.698 | 0.033 | ≥ 39.5 | 86.4 (72%-90%) | 55.6 (36%-61%) | 80 (61%-86%) | 52 (39%-55%) |
| NLR | 0.991 | <0.001 | ≥ 3.5 | 100 (90%-100%) | 94.6 (90%-100%) | 100 (90%-100%) | 93 (90%-199%) |
| 48 hours | |||||||
| D- dimer | 0.779 | 0.003 | ≥ 56.5 | 86.4 (70.4%-90%) | 70.6 (67.1%-77.9%) | 82.2 (79.3%-89.4%) | 72.3 (69.3%-80.3%) |
| Ferritin | 0.747 | 0.009 | ≥ 629 | 81.8 (77.5%-85%) | 59 (53.2%-65%) | 80.6 (77.4%-90.6%) | 62.6 (55%-70%) |
| CRP | 1 | <0.001 | ≥ 0.85 | 90.9 (85%-92%) | 100 (85%-100%) | 90 (85%-92%) | 100 (85%-100%) |
| NLR | 1 | <0.001 | ≥ 5.5 | 95.5 (85%-97%) | 100 (85%-100%) | 94 (85%-96%) | 100 (85%-100%) |
Discussion
In this study, our findings showed that serum D-dimer, NLR, and CRP on admission had a significant role in mortality prediction. The highest one in prediction was NLR, with the highest under the curve and sensitivity (100%), specificity (94.6%), PPV, and NPV at a cut-off ≥ 3.5. Likewise, 48 hours from admission, the serum CRP, ferritin, D-dimer, NLR, and SOFA score after 48 hours had a significant role in the prediction of mortality. Both NLR and D-dimer had the highest area under the curve and sensitivity (95.5 % for NLR vs. 90.9% for D-dimer), specificity (100% for both), PPV, and NPV at a cut-off value of 5.5 and 0.85 for both, respectively. Besides, the vitamin D level after 48 hours had a significant role in predicting mortality at a cut-off ≤ 18. One of the most prevalent test results in hospitalized COVID-19 patients is an elevated D-dimer. In a study of 1099 patients with laboratory-confirmed COVID-19 from over 550 hospitals in China, Guan et al., reported that the Ddimer levels of non-survivors were substantially greater than those of survivors (Median: 2.12 g/ml) [22]. D-dimer levels were significantly higher in deaths associated with Covid-19, as were other abnormal coagulation tests, as previously reported by Ning T., et al [23]. D-dimer levels over 1 g/mL on admission were linked with in-hospital mortality (HR: 18.42, 95% CI: 2.64-128.55), according to research done by Zhou et al [24]. However, the cutoff values for D-dimer in these studies were not well assessed. In patients with COVID-19, an elevated D-dimer suggested a hypercoagulable state that might be caused by several factors. Virus infections often cause a strong proinflammatory response with inadequate regulation of the anti-inflammatory response; therefore, endothelial cell dysfunction and subsequent overproduction of thrombin may be induced [25,26]. By raising blood viscosity and activating a hypoxia-inducible transcription factor-dependent signaling cascade, severe COVID-19 hypoxia may promote thrombosis [27,28]. Risk factors for hypercoagulation or thrombosis among hospitalized patients are similar to those of COVID-19 severity, including invasive treatment, long-term bed rest, underlying illnesses, and older ages [29,30]. Dissections of the lung in critically ill patients with COVID-19 have revealed blockage and microthrombosis accumulation in pulmonary small vessels [31]. At all events, extremely high levels of D-dimer have consistently been linked to adverse outcomes [32,33]. Previously, the lack of specificity has been regarded as a disadvantage of D-dimer [34]. However, low specificity is now considered to be one of its benefits in the assessment of prognosis. Recent research has revealed that NLR levels may have a predictive significance in COVID-19 patients since they were greater in more severe patients [35,36]. The severe COVID-19 patients were more likely to have greater levels of inflammation when they were admitted to the hospital, which is the underlying pathophysiology that supports the clinical use of this biomarker. To identify patients who should be given priority for limited resources, early risk stratification may be made possible by acquiring NLR levels at the time of hospital admission. Onadmission NLR levels were greater in severe and non-survivors of COVID-19 than in non-severe and survivors, according to a recent meta-analysis (SMD 0.88; 95% CI 0.72-1.04; and 1.87; 95% CI 1.25-2.49, respectively). The pooled mortality rate in patients with elevated vs. normal NLR levels was (RR=2.74, 95% CI 0.98-7.66), regardless of the various NLR cut-off values [37]. Two investigations used ROC analysis to identify the best cut-off values. Yan et al. tested the NLR at the 11.75 cutoff point and their findings revealed an AUC value of 0.945, a sensitivity of 97.5%, and a specificity of 78.1% [38]. On the other hand, Cheng et al. showed that at a cut-off value of 7.945, the AUC value was 0.827, the sensitivity was 65.3%, and the specificity was 90.6% [39].
The large range of NLR cutoff values suggests that absolute NLR levels recorded in various populations are not directly comparable and that the ideal cutoff values may vary from population to population. NLR is more practical for clinical use since it is easily collected in standard blood tests compared to other laboratory markers that predict the prognosis of COVID-19, such as erythrocyte sedimentation rate, C-reactive protein, D-dimer levels, and interleukin-6 [40,41]. Moreover, NLR is a cost-effective, near real-time, accessible, low-cost, and simple test, especially for healthcare facilities with limited medical resources [42]. Excessive inflammation owing to infection causes hyperferritinemia, which is linked with hospitalization and a high risk of death and may be used as a diagnostic marker to identify at-risk individuals and direct treatment aimed at reducing inflammation [43-45]. Patients with impaired lung function and elevated serum ferritin levels are more likely to have a bad prognosis after contracting COVID-19. Hemophagocytic lymphohistiocytosis is a documented consequence of viral infection [46, 47]. An elevated ferritin level in the blood may be used as an indicator of viral replication [48, 49]. Patients with severe cases of COVID-19 have also been observed to have elevated ferritin levels owing to cytokine storm and sHLH [50,51]. Macrophages, Kupffer cells, and Hepatocytes are stimulated to release ferritin during the cytokine storm in COVID-19, which results from the rapid production of numerous inflammatory cytokines, including During the cytokine storm in COVID-19, many inflammatory cytokines are rapidly produced, including IFN‐γ, IL‐12, IL‐ 1β, TNF‐α, and IL‐6 [52]. Damage to multiple organs results from an immune response that is out of control, as seen in cases of macrophage activation, thrombotic storm, and hyperferritinemic syndrome. Similar to our findings, Feld et al. demonstrated that increased serum ferritin levels are not reliable indicators of outcomes and do not seem to be suggestive of hemophagocytic lymphohistiocytosis. On receiver operator curve (ROC) analysis, they observed that death was only marginally predicted by admission and maximum ferritin levels, with AUCs of 0.677 and 0.638, respectively. When the analysis was restricted to progressively younger patients, AUCs rose [53]. Higher CRP concentrations have been linked to more severe COVID-19 disease, according to a number of recent data.1,7-12 Nonetheless, the majority of studies were underpowered and did not assess either a dosage response or demographic heterogeneity. CRP levels were related to mortality with an AUC of 0.896 in previous research of 298 patients with COVID-19. 9 Patients with high-sensitivity CRP >5 mg/L were nearly five times more likely to develop ARDS compared to those with lower CRP values, according to recent reports examining the correlation between CRP concentrations and respiratory failure requiring mechanical ventilation.12,17 Multiple studies have found relationships between CRP concentrations and myocardial damage, and CRP is related to extra-pulmonary illness in COVID-19.18-21 Bastug et al., demonstrated that NLR, CRP, and D-dimer had the highest AUC in the ROC analysis in terms of predicting severe illness and mortality (0.861, 0.896, and 0.874, respectively) [54]. Hepatic damage in COVID-19 individuals has been linked to lymphopenia and CRP [55]. A meta-analysis of 25 studies reported less sensitivity and specificity of CRP in predicting COVID-19 severe illness compared to our findings. They showed that a CRP ≥ 10 mg/L has a 51% sensitivity, 88% specificity, and an AUC of 0.84. This difference could be attributed to the different populations and the varied cutoff point used [56]. Serum CRP levels may be useful in predicting poor outcomes in COVID-19, but other variables, including liver injury, blood pressure, cholesterol levels, weight, smoking status, gender, and age may influence these values [57]. The interpretation of the serum CRP level has to take these factors into consideration. In addition, new evidence suggests that blood CRP levels may be used to track how well a patient with COVID-19 is doing [58].
Concerning vitamin D, it has been shown that low vitamin D levels may be associated with SARS-CoV-2 infection and COVID-19-related hospitalization. In COVID-19 patients, there was a very high frequency of hypovitaminosis D (100%) [59]. An Independent and substantial association between low 25(OH)D status (30 ng/mL) and a higher risk of COVID-19 infection was shown in a recent large, real-world, population-based investigation [60]. Low levels of 25(OH)D were also substantially related to an increased risk of hospitalization due to COVID-19 in univariate analysis of the aforementioned sample [60]. The risk of contracting COVID-19 (defined as a positive PCR test result) was also raised in those with vitamin D deficiency, according to the results of a retrospective cohort research conducted at a single center [61]. In addition, a smaller retrospective cohort study indicated that individuals with SARS-CoV-2 PCR positivity had considerably lower blood 25(OH)D levels than those without the infection [62]. Patients with deficient 25(OH)D values (20 ng/mL) had a higher SARS-CoV-2 positivity rate than patients with adequate values (30 ng/mL-34 ng/mL) and those with values 55 ng/mL, according to a retrospective observational analysis conducted in the United States among 191,779 patients with SARS-CoV-2 results performed between midMarch and mid-June 2020 [63].
Conclusion
CRP, NLR, and D-dimer were found to be reliable predictors of COVID-19 outcomes, including critical illness and mortality. Elevated serum ferritin and vitamin D can be used as supplementary predictors but cannot be relied on as independent predictors. The interpretation of these biomarkers should be correlated with many demographic and clinical factors.
Acknowledgment
We acknowledge that our study has some limitations, including the small sample size and the single-center setting, which might hinder the generalizability of our findings. In addition, we did not correlate the study parameters with each other or with the biomarkers of multiple organ failure.
Declarations
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abdel-Ghaffar, Muhammad M., et al. "Prediction of mortality in hospitalized Egyptian patients with Coronavirus disease-2019: A multicenter retrospective study." Plos one, Vol. 17, No. 1, 2022, p. e0262348.
Google Scholar Crossref - Omran, Dalia, et al. "Predictors of severity and development of critical illness of Egyptian COVID-19 patients: A multicenter study." Plos one, Vol. 16, No. 9, 2021, p. e0256203.
Google Scholar Crossref - Infante, Marco, et al. "Hydroxychloroquine in the COVID-19 pandemic era: in pursuit of a rational use for prophylaxis of SARS-CoV-2 infection." Expert review of anti-infective therapy, Vol. 19, No. 1, 2021, pp. 5-16.
Google Scholar Crossref - Liu, Jing, et al. "Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients." EBioMedicine, Vol. 55, 2020.
Google Scholar Crossref - Huang, Chaolin, et al. "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China." The lancet, Vol. 395, No. 10223, 2020, pp. 497-06.
Google Scholar Crossref - Moore, John B., and Carl H. June. "Cytokine release syndrome in severe COVID-19." Science, Vol. 368, No. 6490, 2020, pp. 473-74.
Google Scholar Crossref - Ye, Qing, Bili Wang, and Jianhua Mao. "The pathogenesis and treatment of the Cytokine Storm'in COVID-19." Journal of infection, Vol. 80, No. 6, 2020, pp. 607-13.
Google Scholar Crossref - Zaim, Sevim, et al. "COVID-19 and multiorgan response." Current problems in cardiology, Vol. 45, No. 8, 2020, p. 100618.
Google Scholar Crossref - Zhou, Fei, et al. "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study." The Lancet, Vol. 395, No. 10229, 2020, pp. 1054-62.
Google Scholar Crossref - Tang, Ning, et al. "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia." Journal of thrombosis and haemostasis, Vol. 18, No. 4, 2020, 844-47.
Google Scholar Crossref - Caprio, Massimiliano, et al. "Vitamin D: not just the bone. Evidence for beneficial pleiotropic extraskeletal effects." Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, Vol. 22, No. 1, 2017, pp. 27-41.
Google Scholar Crossref - Fabbri, A., M. Infante, and C. Ricordi. "Editorial-Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections." European Review for Medical Pharmacological Sciences, Vol. 24, No. 7, 2020, pp. 4048-52.
Google Scholar - Infante, Marco, et al. "The role of vitamin D and omega-3 PUFAs in islet transplantation." Nutrients, Vol. 11, No. 12, 2019, p. 2937.
Google Scholar Crossref - Charoenngam, Nipith, and Michael F. Holick. "Immunologic effects of vitamin D on human health and disease." Nutrients, Vol. 12, No. 7, 2020, p. 2097.
Google Scholar Crossref - Infante, Marco, et al. "Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes." Nutrients, Vol. 11, No. 9, 2019, p. 2185.
Google Scholar Crossref - Holick, Michael F. "The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention." Reviews in Endocrine and Metabolic Disorders, Vol. 18, No. 2, 2017, pp. 153-65.
Google Scholar Crossref - Martineau, Adrian R., and Nita G. Forouhi. "Vitamin D for COVID-19: a case to answer?." The Lancet Diabetes & Endocrinology, Vol. 8, No. 9, 2020, pp. 735-36.
Google Scholar Crossref - Infante, Marco, et al. "Low vitamin D status at admission as a risk factor for poor survival in hospitalized patients with COVID-19: an Italian retrospective study." Journal of the American Nutrition Association, Vol. 41, No. 3, 2022, pp. 250-65.
Google Scholar Crossref - Huespe, Iván, et al. "COVID-19 Severity Index: A predictive score for hospitalized patients." Medicina intensiva, Vol. 46, No. 2, 2022, p. 98.
Google Scholar Crossref - Singer, Mervyn, et al. "The third international consensus definitions for sepsis and septic shock (Sepsis-3)." Jama, Vol. 315, No. 8, 2016, pp. 801-10.
Google Scholar Crossref - Prokop, Mathias, et al. "CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19—definition and evaluation." Radiology, Vol. 296, No. 2, 2020, pp. 97-04.
Google Scholar Crossref - Guan, Wei-jie, et al. "Clinical characteristics of coronavirus disease 2019 in China." New England journal of medicine, Vol. 382, No. 18, 2020, pp. 1708-20.
Google Scholar Crossref - Tang, Ning, et al. "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia." Journal of thrombosis and haemostasis, Vol. 18, No. 4, 2020, pp. 844-47.
Google Scholar Crossref - Zhou, Fei, et al. "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study." The Lancet, Vol. 395, No. 10229, 2020, pp. 1054-62.
Google Scholar Crossref - Wong, Jonathan P., et al. "Current and future developments in the treatment of virus-induced hypercytokinemia." Future medicinal chemistry, Vol. 9, No. 2, 2017, 169-78.
Google Scholar Crossref - Levi, Marcel, and Tom van der Poll. "Coagulation and sepsis." Thrombosis Research, Vol. 149, 2017, pp. 38-44.
Google Scholar Crossref - Gupta, Neha, You-Yang Zhao, and Colin E. Evans. "The stimulation of thrombosis by hypoxia." Thrombosis Research, Vol. 181, 2019, pp. 77-83.
Google Scholar Crossref - Tang, Ning, et al. "Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy." Journal of thrombosis and haemostasis, Vol. 18, No. 5, 2020, pp. 1094-99.
Google Scholar Crossref - Harper, P. L., et al. "D‐dimer concentration increases with age reducing the clinical value of the D‐dimer assay in the elderly." Internal medicine journal, Vol. 37, No. 9, 2007, pp. 607-13.
Google Scholar Crossref - Hess, Katharina, and Peter J. Grant. "Inflammation and thrombosis in diabetes." Thrombosis and haemostasis, Vol. 105, No. 6, 2011, pp. 43-54.
Google Scholar Crossref - Zheng, Si-qian, et al. "Recommendations and guidance for providing pharmaceutical care services during COVID-19 pandemic: a China perspective." Research in social and administrative pharmacy, Vol. 17, No. 1, 2021, pp. 1819-24.
Google Scholar Crossref - Zhang, L., et al. "D‐dimer to guide the intensity of anticoagulation in Chinese patients after mechanical heart valve replacement: a randomized controlled trial." Journal of Thrombosis and Haemostasis, Vol. 15, No.10, 2017, pp. 1934-41.
Google Scholar Crossref - Tripodi, Armando. "D-dimer testing in laboratory practice." Clinical chemistry, Vol. 57, No. 9, 2011, pp. 1256-62.
Google Scholar Crossref - Zhang, L., et al. "Use of D‐dimer in oral anticoagulation therapy." International journal of laboratory hematology, Vol. 40, No. 5, 2018, pp. 503-07.
Google Scholar Crossref - Lagunas‐Rangel, Francisco A. "Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis." Journal of medical virology, 2020.
Google Scholar Crossref - Liu, Yuwei, et al. "Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19." Journal of Infection, Vol. 81, No. 1, 2020, pp. 6-12.
Google Scholar Crossref - Simadibrata, Daniel M., et al. "Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: A meta-analysis." The American journal of emergency medicine, Vol. 42, 2021, pp. 60-69.
Google Scholar Crossref - Yan, Xisheng, et al. "Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross‐sectional study." Journal of medical virology, Vol. 92, No. 11, 2020, pp. 2573-81.
Google Scholar Crossref - Zhou, Jing, et al. "Clinical features predicting mortality risk in older patients with COVID-19." Current Medical Research and Opinion, Vol. 36, No. 11, 2020, pp. 1753-59.
Google Scholar Crossref - Ponti, G., M. Maccaferri, and C. Ruini. "Biomarkers associated with COVID-19 disease progression" Crit Rev Clinical Lab Science, 2020, pp. 1-11.
Google Scholar Crossref - Simadibrata, Daniel Martin, and Anna Mira Lubis. "D-dimer levels on admission and all-cause mortality risk in COVID-19 patients: a meta-analysis." Epidemiology and Infection, Vol. 148, 2020.
Google Scholar Crossref - Guo, Jinan, et al. "Prognostic role of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in prostate cancer: a meta-analysis of results from multivariate analysis." International Journal of Surgery, Vol. 60, 2018, pp. 216-23.
Google Scholar Crossref - Kernan, Kate F., and Joseph A. Carcillo. "Hyperferritinemia and inflammation." International immunology, Vol. 29, No. 9, 2017, pp. 401-9.
Google Scholar Crossref - Bennett, Tellen D., et al. "Very high serum ferritin levels are associated with increased mortality and critical care in pediatric patients." Pediatric Critical Care Medicine, Vol. 12, No. 6, 2011, pp. 233-36.
Google Scholar Crossref - Carcillo, Joseph A., et al. "A systemic inflammation mortality risk assessment contingency table for severe sepsis." Pediatric Critical Care Medicine, Vol. 18, No. 2, 2017, pp. 143-50.
Google Scholar Crossref - Fu, Sha, et al. "Virologic and clinical characteristics for prognosis of severe COVID-19: a retrospective observational study in Wuhan, China." MedRxiv, 2020.
Google Scholar Crossref - Mehta, Puja, et al. "COVID-19: consider cytokine storm syndromes and immunosuppression." The lancet, Vol. 395, No. 10229, 2020, pp. 1033-34.
Google Scholar Crossref - Baraboutis, Ioannis G., et al. "Initial real-life experience from a designated COVID-19 centre in Athens, Greece: a proposed therapeutic algorithm." SN Comprehensive Clinical Medicine, Vol. 2, No. 6, 2020, pp. 689-93.
Google Scholar Crossref - Li, Yanlei, et al. "Retrospective analysis of laboratory testing in 54 patients with severe-or critical-type 2019 novel coronavirus pneumonia." Laboratory investigation, Vol. 100, No. 6, 2020, pp. 794-800.
Google Scholar Crossref - Velavan, Thirumalaisamy P., and Christian G. Meyer. "Mild versus severe COVID-19: laboratory markers." International Journal of Infectious Diseases, Vol. 95, 2020, pp. 304-07.
Google Scholar Crossref - Giamarellos-Bourboulis, Evangelos J., et al. "Complex immune dysregulation in COVID-19 patients with severe respiratory failure." Cell host and microbe, Vol. 27, No. 6, 2020, pp. 992-1000.
Google Scholar Crossref - Torti, Frank M., and Suzy V. Torti. "Regulation of ferritin genes and protein." Blood, Vol. 99, No. 10, 2002, pp. 3505-16.
Google Scholar Crossref - Feld, Jonathan, et al. "Ferritin levels in patients with COVID‐19: a poor predictor of mortality and hemophagocytic lymphohistiocytosis." International journal of laboratory hematology, Vol. 42, No. 6, 2020, pp. 773-79.
Google Scholar Crossref - Bastug, Aliye, et al. "Clinical and laboratory features of COVID-19: Predictors of severe prognosis." International immunopharmacology, Vol. 88, 2020.
Google Scholar Crossref - Li, Lu, et al. "Risk factors related to hepatic injury in patients with corona virus disease 2019." MedRxiv, 2020.
Google Scholar Crossref - Huang, Ian, et al. "C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis." Therapeutic advances in respiratory disease, Vol. 14, 2020.
Google Scholar Crossref - Sproston, Nicola R., and Jason J. Ashworth. "Role of C-reactive protein at sites of inflammation and infection." Frontiers in immunology, Vol. 9, 2018, p. 754.
Google Scholar Crossref - Li, Huan, et al. "Serum Amyloid A is a biomarker of severe Coronavirus Disease and poor prognosis." Journal of Infection, Vol. 80, No. 6, 2020, pp. 646-55.
Google Scholar Crossref - Carpagnano, Giovanna Elisiana, et al. "Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19." Journal of endocrinological investigation, Vol. 44, No. 4, 2021, pp. 765-71.
Google Scholar Crossref - Merzon, Eugene, et al. "Low plasma 25 (OH) vitamin D level is associated with increased risk of COVID‐19 infection: an Israeli population‐based study." The FEBS journal, Vol. 287, No. 17, 2020, pp. 3693-02.
Google Scholar Crossref - Meltzer, David O., et al. "Association of vitamin D status and other clinical characteristics with COVID-19 test results." JAMA network open, Vol. 3, No. 9, 2020.
Google Scholar Crossref - Brandão, Cynthia M. Álvares, et al. "No association between vitamin D status and COVID-19 infection in São Paulo, Brazil." Archives of endocrinology and metabolism, Vol. 65, 2021, pp. 381-85.
Google Scholar Crossref - Kaufman, Harvey W., et al. "SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels." PloS one, Vol. 15, No. 9, 2020.
Google Scholar Crossref