Research - International Journal of Medical Research & Health Sciences ( 2022) Volume 11, Issue 3
Long-term Efficacy snd Safety of Bosentan in Patients with Digit al Ulcers Related to Systemic Sclerosis
Laura Groseanu1,2, Cristina Nita1, Oana Naiman2, Violeta Bojinca1,2*, Andra Balanescu1,2, Daniela Opris-Belinski1,2, Denisa Predeteanu1,2, Florian Berghea1,2, Ioana Saulescu1,2, Sanziana Daia-Iliescu1,2, Diana Mazilu1,2, Andreea Borangiu1,2, Cosmin Constantinescu1,2, Mihai Abobului1,2 and Ruxandra Ionescu1,22”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
Violeta Bojinca, Department of Internal Medicine and Rheumatology, Sfanta Maria Clinical Hospital, Bucharest, Romania, Email: violetaclaudiabojinca@gmail.com
Received: 09-Mar-2022, Manuscript No. ijmrhs-22-56672 (M); Editor assigned: 11-Mar-2022, Pre QC No. ijmrhs-22-56672 (P); Reviewed: 18-Mar-2022, QC No. ijmrhs-22-56672 (Q); Revised: 20-Mar-2022, Manuscript No. ijmrhs-22-56672 (R); Published: 31-Mar-2022
Abstract
Objective: Two pivotal studies, RAPIDS-1 and RAPIDS-2 (RAndomized, double-blind, Placebo-controlled study with bosentan on healing and prevention of Ischemic Digital ulcers in patients with systemic Sclerosis) revealed that Bosentan reduces the development of new Digital Ulcers (DUs) in patients with Systemic Sclerosis (SSc). However, data regarding the long-term use of this dual endothelin antagonist receptor in the treatment of DUs is scarce. Methods: We conducted a prospective observational case-control study, between 2014 and 2020 that enrolled 80 SSc patients with at least one active DU at baseline compatible with a vascular etiology or recurrent DUs within the previous 3 months. DUs number, patients’ subjective perception of DUs’ pain and/or Raynaud’s phenomenon, nail fold video-capillaroscopy, and Health Assessment Questionnaire (HAQ) were reassessed every 6 months, for up to 60 months after treatment initiation. Results: At week 24, bosentan therapy was associated with a significant reduction in the number of DUs (p<0.001) and significant improvement of quality of life (p<0.001), patients’ subjective perception of DUs’ pain (p<0.001) and Raynaud’s phenomenon (p<0.001) compared to baseline and benefits were maintained up to month 60. Long-term use of bosentan also improved the Microangiopathy Evolution Score (MES) and the difference was statistically significant between bosentan-treated and the control group (p=0.005). Accelerated development of new DUs was described 6 months after temporarily stopping bosentan. Following the re-initiation of treatment, the mean number of DUs rapidly decreased. Conclusion: Bosentan has long-term efficacy in DUs prevention in SSc patients, a tolerable safety profile, and might improve microvascular remodeling.
Keywords
Systemic sclerosis, Digital ulcers, Bosentan, Efficacy, Safety, Long-term
Introduction
SSc is a rare connective tissue disease in which vascular dysfunction, tissue fibrosis, and immune dysregulation are key events [1]. The near-universal initial clinical manifestation of SSc is the Raynaud phenomenon and associated digital vasculopathy, indicating that endothelial dysfunction is an early, if not primary event in SSc pathogenesis [2,3]. DUs are a serious manifestation of SSc-related digital vasculopathy and are a marker for disease severity [4]. They occur as a result of structural vascular disease as well as vasospasm [5].
Up to 30%-50% of SSc patients will suffer from at least one ischemic digital ulcer; 66% of the patient have recurrent ulcers throughout the natural history of an SSc patient [5,6]. Moreover, patients with SSc and DUs develop internal organ involvement 2 to 3 years earlier than those without DUs [7,8]. Complications of DUs include irreversible tissue loss, as well as other significant complications such as osteomyelitis, gangrene, and amputation [9]. In addition, the degree of functional impairment is considered as well. Patients with chronic, recurrent DUs are prone to experience the highest disease burden and should be accordingly recognized and intensively managed [10]. These ischemic lesions decrease patients’ quality of life because they are painful, disabling, and frequently lead to hospitalization [11].
Bosentan, an oral inhibitor of endothelin-1, is widely used for the prevention of new DUs in SSc [12]. Two large randomized clinical trials showed the efficacy of bosentan in this setting and are the reason for its approval for this indication by regulatory agencies [13,14]. However, there are insufficient data about the long-term use of this drug. Single reports small retrospective studies and two prospective studies, all have reported the efficacy of bosentan for SSc-associated DUs’ prevention [15-22]. Although there have been considerable advances in the management of SSc-related vasculopathy, the authors noted that large trials can provide a superior approach by analyzing a more precise estimate of the treatment effect. Moreover, there is a need to consider moving away from short-term trials to longer-term, event-driven trials with composite endpoints that better reflect the ultimate goals of reducing morbidity and mortality rates of SSc-related DUs.
Because of the short-term beneficial results observed with bosentan in patients with SSc and digital ulcers, we aimed to investigate the potential benefit and safety of long-term treatment in a larger group of patients treated with bosentan.
Materials and Methods
Study Design
This was a prospective, observational, case-control study conducted in 2 university hospitals in Bucharest from September 2014 to December 2020, which enrolled 80 SSc patients with DUs, who received bosentan therapy. All 80 patients were assigned to receive bosentan according to standard recommendations (62.5 mg twice daily for 4 weeks and 125 mg twice daily till the end of study up to month 60). All patients were evaluated at baseline and 6 months intervals. The control group included 20 patients with SSc-related DUs, who did not receive bosentan treatment. The two groups were well matched concerning demographic features and baseline disease characteristics. The sample size was calculated with an error of 0.05 and a power of 80%.
Selection of Patients
Adults (≥ 18 years of age) were eligible for inclusion if they had a diagnosis of SSc based on ACR/EULAR criteria and at least one active DU compatible with a vascular etiology or recurrent DUs in the last 3 months [23]. They were also required to have a documented history of vasodilators such as calcium antagonists, prostacyclins, or PDE-5 inhibitors providing inadequate control of the DUs. All patients signed informed consent.
Assessing Vascular Damage Progression
Nailfold Videocapillaroscopy (NVC) was performed with CAM1Capiscope with a 200 × magnification lens. All assessments were performed by the same rheumatologist, using qualitative and semi-quantitative scoring systems. All images were scored for each patient at baseline and then every 6 months. Capillaroscopic findings were described following qualitative classification of scleroderma microangiopathy damage described by Cutolo as early, active and late patterns [24]. A semiquantitative rating scale to score the altered microvascular parameters was adopted (score 0-3) [25]. The Microangiopathy Evolution Score (MES) (sum of three scores: loss of capillaries, disorganization of the microvascular array, and capillary ramifications) was also performed [26].
Outcome Measures
Patients were evaluated on an outpatient basis every 6 months. The primary outcome of the study was the longterm efficacy and safety of bosentan. Secondary outcomes included the evolution of the Raynaud phenomenon, the NVC dynamic changes, and health-related quality of life during bosentan treatment. The severity of RP and DUs were measured using visual analog scales and the impact of DUs on quality of life through the Health Assessment Questionnaire (HAQ). Safety was assessed based on recorded adverse events and laboratory measures (cell blood count, Aspartate Transaminase (AST), and Alanine Transaminase (ALT) according to standard recommendations).
Statistical Analysis
The statistical analysis was performed with SPSS 27.0 Quantitative variables were described with mean ± Standard Deviation (SD) or median and interquartile range for normally distributed or non-normally distributed continuous data, respectively. Qualitative variables were described with frequencies and percentages. Levene’s test for equality of variance was performed to compare the paired groups. Treatment efficacy is shown as the mean difference from baseline of all patients remaining at the assessment point in time. The difference between efficacy measures at followup visits and baseline was tested with Wilcoxon’s signed-rank test. p<0.05 was considered statistically significant.
Result
The current study enrolled a total of 80 patients (64 females and 16 males), with a mean age of 52.6 (± 12) years, 50% of patients with diffuse cutaneous involvement, most of them with a late NVC scleroderma pattern (54/80) and with antitopoisomerase-1 antibodies (48/80). 55% of the patients had interstitial lung disease, 16% of the patients had pulmonary hypertension and 5% had scleroderma renal crisis. The average disease duration before the bosentan therapy was 7.8 (± 7.9) years.
The median duration of treatment was 25.95 (± 19.4) months. While 16.25% of patients completed 6 months of therapy, most of the patients (18.75%) completed 12 months of treatment, but only 13.75% completed the entire duration of the study of 60 months. 28.75% of the patients had temporarily stopped bosentan due to logistic issues or to increased liver enzymes according to recommendations. The mean duration of the pause was 6.9 (2.2) months; 69.56% of those patients restarted bosentan and were re-evaluated every 6 months.
The number of DUs at baseline was 4.55 (± 2.8). MES was 5.1 (2.19), mean VAS for DUs was 78.6 (46.4), mean VAS for Raynaud was 71.7 (46.32), and mean HAQ at baseline was 1.62 (0.55).
DUs Assessment
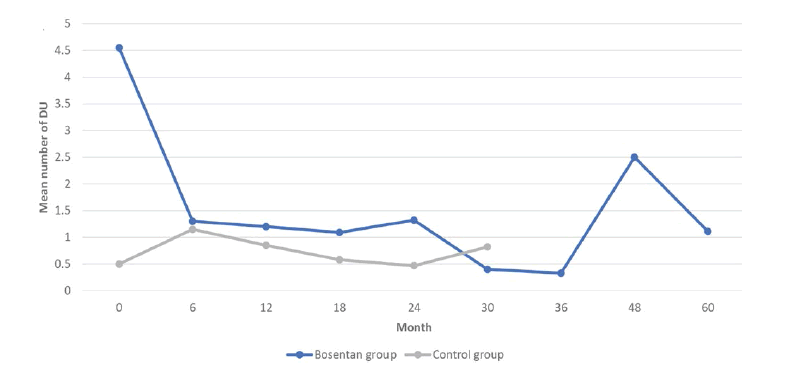
Patients receiving bosentan had a clinically significant reduction in the mean number of DUs (p<0.001) after the first 6 months of treatment. A plateau was reached after the first 6 months since there were no significant differences between the other 2 consecutive follow-up evaluations (Figure 1). However, the benefits of Bosentan persisted until the end of the study when compared to baseline (p<0.001).
Digital Ulcers (DU)
VAS for DUs decreased significantly after 6 months (p<0.001) and the effect was kept till the end of the study (p<0.001) (Table 1). VAS for Raynaud also decreased significantly after the first follow-up (p<0.001) (Table 1). As a consequence of the decrease in the total number of DUs and Raynaud’s severity, compared to baseline, a significant improvement of life quality was noticed in the first 3 years of treatment (p<0.001).
| Parameters | Baseline | 6 months | 12 months | 18 months | 24 months | 30 months | 36 months | 48 months | 60 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p-value | Mean ± SD | p-value | Mean ± SD | p-value | Mean ± SD | p-value | Mean ± SD | p-value | Mean ± SD | p-value | Mean ± SD | p-value | Mean ± SD | p-value | |
| No DUs | 4.55 (± 2.8) | 3.4 (± 2.0) | 0.00 | 0.2 (± 1.8) | 0.48 | 0.3 (± 2.2) | 0.44 | 0.1 (± 1.0) | 0.60 | 0.2 (± 1.3) | 0.32 | -0.1 (± 0.8) | 0.50 | -0.9 (± 3.5) | 0.27 | 1.2 (± 3.9) | 0.33 |
| VAS for Raynaud | 71.7 (± 46.32) | 33.7 (± 28.7) | 0.00 | 6.5 (± 18.3) | 0.00 | 4.8 (± 15.3) | 0.14 | 0.4 (± 13.7) | 0.91 | -0.3 (± 11.7) | 0.92 | -2.6 (± 11.3) | 0.51 | -3.8 (± 11.6) | 0.14 | 4.9 (± 15.7) | 0.32 |
| VAS for DUs | 78.6 (± 46.4) | 48.0 (± 28.6) | 0.00 | 2.4 (± 22.3) | 0.00 | 6.7 (± 16.9) | 0.00 | -4.7 (± 22.5) | 0.00 | 6.1 (± 29.1) | 0.00 | 0.8 (± 23.2) | 0.00 | -4.6 (± 20.1) | 0.00 | -0.6 (± 21.8) | 0.00 |
| HAQ | 1.62 (± 0.55) | 0.4(± 0.5) | 0.00 | 0.0 (± 0.4) | 0.80 | 0.0 (± 0.4) | 0.66 | 0.0 (± 0.4) | 0.89 | 0.0 (± 0.5) | 0.99 | 0.0 (± 0.2) | 0.78 | 0.2 (± 0.7) | 0.23 | 0.0 (± 0.5) | 0.94 |
| Mean MES | 5.1 (± 2.19) | -0.4 (± 0.8) | 0.01 | -0.4 (± 0.7) | 0.01 | -0.4 (± 0.7) | 0.05 | -0.2 (± 0.4) | 0.16 | 0.2 (± 0.4) | 0.16 | -0.6 (± 0.7) | 0.05 | 0.5 (± 0.7) | 0.50 | -2.5 (± 0.7) | 0.12 |
| DUs: Digital Ulcers; VAS: Visual Analog Scale; HAQ: Health Assessment Questionnaire; MES: Microangioapthy Evolution Score; SD: Standard Deviation; mean: mean difference from baseline; (*p<0.05) | |||||||||||||||||
Microvascular Alterations
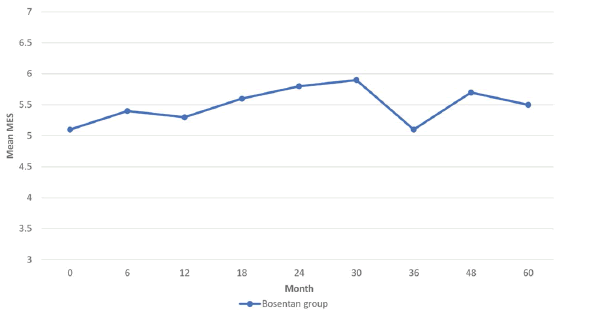
The benefits of bosentan on the progression of microangiopathy had not been clearly defined during the first 2 years of treatment, but on the other hand, beyond that period, no significant increase of MES between 2 consecutive evaluations could be noticed until the end of the study (Table 1). Overall, based on the fact that MES values between baseline and month 60 are not statistically different, we could conclude that bosentan slows down the progression of endothelial dysfunction. The difference was statistically significant when compared to the control group, but only for the first 18 months of treatment (p<0.001) (Figure 2).
Microangiopathy Evolution Score (MES)
MES values were significantly higher in the bosentan treated group compared to control up to month 18 (p<0.001), but after month 24 mean values are comparable between the 2 groups. This strengthens the idea that long-term treatment with bosentan could slow down the progression of microangiopathy.
The Effect of Temporarily Stopping the Bosentan Treatment
23 patients (28.75%) discontinued bosentan therapy due to logistic reasons. The median duration of discontinuation was 6.9 months (± 28.75). A significant increase of new DUs was described 6 months after (p=0.025). Following reinitiation of bosentan, the mean number of DUs has rapidly decreased (p=0.008). There was no significant difference regarding the number of new DUs between patients who temporarily discontinued bosentan for 6 or 12 months. Subgroup analysis revealed the same pattern.
Safety and Tolerability
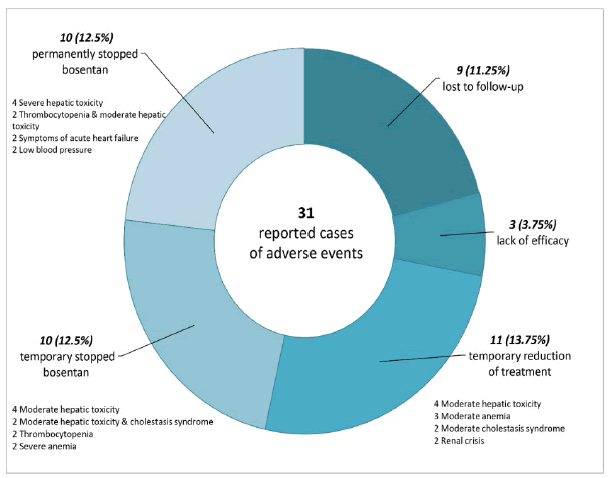
Bosentan was stopped due to lack of efficacy in 3 (3.75%) cases (significant increase of new DUs number at follow-up requiring digital amputation).
The most frequent adverse events in the bosentan-treated group are shown in Figure 3. Adverse events led to premature discontinuation of the study medication in 10 (12.5%) patients. In 13.75% of the patients, the bosentan dose was reduced and in 12.5% of the cases treatment was temporarily stopped and then restarted.
The most frequent adverse events leading to withdrawal were abnormal liver tests (15% of the patients) but in only 5% of the cases, it was permanently interrupted (increased hepatic aminotransferase levels more than eight times the upper limit of normal). In 2 cases treatment was withdrawn because of severe thrombocytopenia, in the other 2 because of dyspnea worsening and low blood pressure.
13.75% of patients required a reduction of the dose because of increased hepatic aminotransferase levels less than eight times the upper limit of normal (4 cases), cholestasis (2 cases), renal crisis (2 cases), anemia (3 cases). In 12.5% of the cases treatment was temporarily stopped (elevated liver enzymes 5%, cholestasis-2.5%, thrombocytopenia 2.5%, anemia-2.5%).
9 patients were lost to follow-up. 5% of subjects died within the first year after initiating bosentan and 5% during the second year after treatment onset. None of the reported deaths have been considered related to study medication, but rather to the progression of the treated concomitant diseases.
Conclusion
The present data establish the long-term efficacy of bosentan in decreasing the overall number of DUs (present at baseline and/or new) in patients with SSc and improving patients’ evaluation and quality of life. The beneficial effect of bosentan persisted throughout the study but was most evident in the first 6 months of treatment. Statistical analysis also showed a trend in slowing the microangiopathy evolution score from baseline to end of therapy. Further studies assessing the clinical usefulness of our data are needed to validate their application in routine use, preventive or early therapeutic strategies.
References
- Van Den Hoogen, Frank, et al. "2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against rheumatism collaborative initiative." Arthritis & Rheumatism, Vol. 65, No. 11, 2013, pp. 2737-47.
Google Scholar Crossref - Prescott, Richard J., et al. "Sequential dermal microvascular and perivascular changes in the development of scleroderma." The Journal of Pathology, Vol. 166, No. 3, 1992, pp. 255-63.
Google Scholar Crossref - Kahaleh, Bashar, O. Meyer, and R. Scorza. "Assessment of vascular involvement." Clinical and Experimental Rheumatology, Vol. 21, No. 3, 2003, pp. S9-14.
Google Scholar Crossref - Steen, V., et al. "Digital ulcers: Overt vascular disease in systemic sclerosis." Rheumatology, Vol. 48, No. suppl_3, 2006, pp. iii19-24.
Google Scholar Crossref - Hughes, Michael, et al. "Consensus best practice pathway of the UK Scleroderma Study Group: Digital vasculopathy in systemic sclerosis." Rheumatology, Vol. 54, No. 11, 2015, pp. 2015-24.
Google Scholar Crossref - Matucci-Cerinic, Marco, et al. "Elucidating the burden of recurrent and chronic digital ulcers in systemic sclerosis: Long-term results from the DUO Registry." Annals of the Rheumatic Diseases, Vol. 75, No. 10, 2016, pp. 1770-76.
Google Scholar Crossref - Sunderkotter, C., et al. "Comparison of patients with and without digital ulcers in systemic sclerosis: Detection of possible risk factors." British Journal of Dermatology, Vol. 160, No. 4, 2009, pp. 835-43.
Google Scholar Crossref - Mihai, Carina, et al. "Digital ulcers predict a worse disease course in patients with systemic sclerosis." Annals of the Rheumatic Diseases, Vol. 75, No. 4, 2016, pp. 681-86.
Google Scholar Crossref - Hachulla, Eric, et al. "Natural history of ischemic digital ulcers in systemic sclerosis: Single-center retrospective longitudinal study." The Journal of Rheumatology, Vol. 34, No. 12, 2007, pp. 2423-30.
Google Scholar Crossref - Abraham, Shawn, and Virginia Steen. "Optimal management of digital ulcers in systemic sclerosis." Therapeutics and Clinical Risk Management, Vol. 11, 2015, pp. 939-47.
Google Scholar Crossref - Mouthon, Luc, et al. "Impact of digital ulcers on disability and health-related quality of life in systemic sclerosis." Annals of the Rheumatic Diseases, Vol. 69, No. 01, 2010, pp. 214-17.
Google Scholar Crossref - Price, Sarah. "Bosentan for SSc: Long-term outcomes in PAH and efficacy for Raynaud phenomenon." Nature Reviews Rheumatology, Vol. 6, No. 3, 2010, p. 117.
Google Scholar Crossref - Korn, J. H., et al. "Digital ulcers in systemic sclerosis: Prevention by treatment with bosentan, an oral endothelin receptor antagonist." Arthritis & Rheumatism, Vol. 50, No. 12, 2004, pp. 3985-93.
Google Scholar Crossref - Matucci-Cerinic, Marco, et al. "Bosentan treatment of digital ulcers related to systemic sclerosis: Results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial." Annals of the Rheumatic Diseases, Vol. 70, No. 1, 2011, pp. 32-38.
Google Scholar Crossref - Chamaillard, Melanie, et al. "Bosentan as a rescue therapy in scleroderma refractory digital ulcers." Archives of Dermatology, Vol. 143, No. 1, 2007, pp. 115-26.
Google Scholar Crossref - Tillon, J. "Successful treatment of systemic sclerosis-related digital ulcers and sarcoidosis with endothelin receptor antagonist (bosentan) therapy." British Journal of Dermatology, Vol. 154, 2006, pp. 1000-02.
Google Scholar Crossref - Humbert, M., and J. Cabane. "Successful treatment of systemic sclerosis digital ulcers and pulmonary arterial hypertension with endothelin receptor antagonist bosentan." Rheumatology, Vol. 42, No. 1, 2003, pp. 191-93.
Google Scholar Crossref - Launay, David, et al. "Bosentan for treatment of active digital ulcers in patients with systemic sclerosis." Presse Medicale (Paris, France: 1983), Vol. 35, No. 4, 2006, pp. 587-92.
Google Scholar Crossref - Funauchi, Masanori, et al. "Effects of bosentan on the skin lesions: An observational study from a single center in Japan." Rheumatology International, Vol. 29, No. 7, 2009, pp. 769-75.
Google Scholar Crossref - Riccardi, M. T., et al. "Treatment of digital ulcers in systemtic sclerosis with endothelin-1 receptor antagonist (bosentan)." Rheumatism, Vol. 59, No. 2, 2007, pp. 135-39.
Google Scholar Crossref - Tsifetaki, Niki, et al. "Bosentan for digital ulcers in patients with systemic sclerosis: A prospective 3-year followup study." The Journal of Rheumatology, Vol. 36, No. 7, 2009, pp. 1550-51.
Google Scholar Crossref - Garcia, de la Pena-Lefebvre P., et al. "Long-term experience of bosentan for treating ulcers and healed ulcers in systemic sclerosis patients." Rheumatology, Vol. 47, No. 4, 2008, pp. 464-66.
Google Scholar Crossref - Johnson, Sindhu R. "New ACR EULAR guidelines for systemic sclerosis classification." Current Rheumatology Reports, Vol. 17, No. 5, 2015, pp. 1-8.
Google Scholar Crossref - Cutolo, Maurizio, Alberto Sulli, and Vanessa Smith. "How to perform and interpret capillaroscopy." Best Practice & Research Clinical Rheumatology, Vol. 27, No. 2, 2013, pp. 237-48.
Google Scholar Crossref - Smith, Vanessa, et al. "Nailfold capillaroscopy for day-to-day clinical use: Construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions." Annals of the Rheumatic Diseases, Vol. 70, No. 1, 2011, pp. 180-83.
Google Scholar Crossref - Sulli, Alberto, et al. "Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients." Annals of the Rheumatic Diseases, Vol. 67, No. 6, 2008, pp. 885-87.
Google Scholar Crossref - Dhillon, Sohita. "Bosentan: A review of its use in the management of digital ulcers associated with systemic sclerosis." Drugs, Vol. 69, No. 14, 2009, pp. 2005-25.
Google Scholar Crossref - Groseanu, L., et al. "AB0779 Long term follow-up of a systemic sclerosis group treated with bosentan." Annals of the Rheumatic Diseases, Vol. 77, No. 2, 2018, pp. 1523-24.
Google Scholar Crossref - Denton, Christopher P., et al. "Long-term effects of bosentan on quality of life, survival, safety and tolerability in pulmonary arterial hypertension related to connective tissue diseases." Annals of the Rheumatic Diseases, Vol. 67, No. 9, 2008, pp. 1222-28.
Google Scholar Crossref - Guiducci, S., et al. "Bosentan fosters microvascular de-remodelling in systemic sclerosis." Clinical Rheumatology, Vol. 31, No. 12, 2012, pp. 1723-25.
Google Scholar Crossref