Review - International Journal of Medical Research & Health Sciences ( 2022) Volume 11, Issue 10
Lung Metastasectomy: Our Experience, Review of Literature and Discussion
Flavio Colaut1*, Tommaso Stecca1, Adriana Di Giacomo1, Francesca Baciorri2, Micaela Romagnoli3, Aurelio Piazza1 and Marco Massani12Department of Pathology, Ca Foncello Hospital, piazza ospedale, Treviso, Italy
3Department of Pulmonology, Ca Foncello Hospital, piazza ospedale, Treviso, Italy
Flavio Colaut, Department of General Surgery and Thoracic, Ca Foncello Hospital, piazza ospedale, 1, 31100, 2 Trevi, Italy, Email: flavio.colaut@aulss2.veneto.it
Received: 07-Oct-2022, Manuscript No. ijmrhs-22-76670; Editor assigned: 10-Oct-2022, Pre QC No. ijmrhs-22-76670 (PQ); Reviewed: 16-Oct-2022, QC No. ijmrhs-22-76670 (Q); Revised: 18-Oct-2022, Manuscript No. ijmrhs-22-76670 (R); Published: 30-Oct-2022
Abstract
Lung metastasectomy became a promising surgical approach to treat different types of cancer. It is believed nowadays that lung localization, after radical removal of the primitive neoplasm, is not to be considered a systemic spread of cancer, but somewhat we can define” organ disease”. So the question is not to use a local therapy to treat a systemic disease but to treat radically an organ with the disease. According to most of the Authors, overall survival in our series is 37% at 5 years. No mortality nor perioperative morbidity has been reported. Most of our interventions were open access, both because of gross disease both to feel lesions. Surgery for lung metastases is safe and should be planned within a multidisciplinary approach because the benefit of such an aggressive surgery is limited to selected subgroups of patients only.
Keywords
Metastasectomy, Lung surgery, Survival
INTRODUCTION
Surgery for lung metastases is a clinical approach to ameliorating oncologic therapy of different types of cancer. [1,2]. If it is widely demonstrated that the prognosis of patients with no treated lung metastases is dismal, Literature has reported encouraging results as regards the survival of patients submitted lung metastasectomy [3,4]. In most cases, surgery represents an integrated oncologic treatment, which includes other antineoplastic strategies,particularly chemotherapy: in fact, surgery associated with chemotherapy seems to improve the outcome in terms of survival, since surgery and chemo permit respectively local and systemic control of cancer [5]. Solitary metastases to the lung, once primitive cancer has been removed, are not to be considered systemic dissemination of the disease, but just a “disease of organ” and represent the first step of an oncologic pathway that will drive to complete neoplastic substitution of the organ tissue [6].
This is why many Authors have proposed a more diffusive use of surgery to treat lung metastases; of course, improving anesthetic and operative proceedings allowed to perform this kind of surgery very safely, with perioperative mortality and morbidity inferior to surgery for lung cancer [7-14]. Nonetheless, some Authors find that lung resection for metastases is not supported by strong evidence [15].
There are some aspects of surgical technique and management that represent objects for discussion among surgeons who deal with this kind of surgery: the main questions are the choice of the correct surgical approach, the type of lung resection to be performed, and eventually the correct surgical approach to remove bilateral lung metastases [16-24]
Objectives
The study aims to evaluate our series from a retrospective point of view and to present the data. Also, a comparison with the Literature has been done.
Materials and Method
From 1998 to 2020 81 patients with lung metastases have been submitted surgery and represented 3.25 % of all interventions performed in our unit in this period; 47 were male (58%) 34 female (42%), mean age was 62 years (18 years to 81 years). Inclusion criteria were: primitive cancer under control, resectable lung nodule/es, no metastatic nodules in extrathoracic sites or single extra thoracic lesions suitable for treatment ,disease - free interval of at least 24 months (median disease free interval was 35.5 months) and patient fit for surgery. Primitive cancer was: colo-rectal 36 patients (44.4%), kidney 12 patients(14.8 %), skin melanoma 8 patients (9.9%), sarcoma 7 patients (8.6%), breast 6 patients (7.4%), endometrial 3 patients (3.7%), cholangiocarcinoma 2 patients (2.5%), thyroid 2 patients (2.5%), larynx 1 patient (1.2%), melanoma of the eye 1 patient (1.2%), adrenal gland 1 patient (1.2%). Two patients with not found type of primitive tumor (Table 1).
| Histology | Frequency (n) | % |
|---|---|---|
| Colo-rectal | 36 | 44.4% |
| Kidney | 12 | 14.8% |
| Melanoma of the skin | 8 | 9.9% |
| Sarcoma | 7 | 8.6% |
| Breast | 6 | 7.4% |
| Endometrial | 3 | 3.7% |
| Cholangio carcinoma | 2 | 2.5% |
| Unknown | 2 | 2.5% |
| Thyroid | 2 | 2.5% |
| Larynx | 1 | 1.2% |
| Melanoma of the eye | 1 | 1.2% |
| Adrenal gland | 1 | 1.2% |
| Total | 81 | 100.0% |
On histogenic-basis criteria, patients have been divided into 4 groups (Table 2).
| Histotype | Frequency (n) | % |
|---|---|---|
| Epithelial | 62 | 78.50% |
| Melanocytic | 9 | 11.50% |
| Mesenchymal | 7 | 8.80% |
| Endocrine | 1 | 1.20% |
| Total | 79 | 100,0% |
• Epithelial: 62 patients [36 adenocarcinomas of the colon, 2 cholangiocarcinomas, 3 carcinomas of the endometrium, 1 squamocellular cancer of the larynx, 2 follicular cancer of the thyroid, 12 cancer of the kidney, 6 lobular cancer of breast] (78.5%).
• Melanocytic: 9 patients [8 Melanomas of the skin, 1 Melanoma of the eye] (11.5%).
• Mesenchymal: 7 patients [sarcomas].
• Endocrine: 1 patient [adrenal gland].
Surgical approach incisions were 57 thoracotomies (70.4%), and 22 VATS (27.2%); one case has been converted to thoracotomy, and one case remains unclassified.
In our series, the so relevant percentage of thoracotomies (70.4%) is due to the need to deal with gross tumor masses, which to be removed required major anatomic resections or to deal with lesions whose exact detection required finger palpation. No one patient has been re-operated (Table 3).
| Access | Frequency (n) | % |
|---|---|---|
| Thoracotomy | 57 | 70.4% |
| Videothoracoscopy converted to thoracotomy | 1 | 1.2% |
| Videothoracoscopy | 22 | 27.2% |
| n.a. | 1 | 1.2% |
| Total | 81 | 100.0% |
Type of resection were: enucleoresection in 1 case (1.2%), 31 wedge resection in 31 cases (38.3%), double wedge resection in in 7 cases (8.6%), wedge resection+lobectomy in 3 cases (3.7 %), double wedge resction+lobectomy in 1 case (1.2%), lobectomy in 32 cases (39.5%), bilobectomy in 1 case (1.2%), pneumonectomy in 2 cases (2.5%), biopsy in 2 cases (2.5%) and in 1 case it was not possible to define the intervention
Sixty-seven patients (83.75%) had single metastasis (32 lobectomies, 31 wedge resections, 2 pneumonectomies, 1 lobectomy, 1 enucleoresection), while 13 patients (16.25%) had multiple metastasis (7 double wedge resections, 3 lobectomies+wedge resection, 1 lobectomy+double wedge resection, 2 biopsies). None of the patients presented bilateral metastatic lung nodules (Table 4).
| Technique | Frequency (n) | % |
|---|---|---|
| Lobectomy | 32 | 39.5% |
| Wedge resection | 31 | 38.3% |
| Double wedge resection | 7 | 8.6% |
| Lobectomy+wedge resection | 3 | 3.7% |
| Pneumectomy | 2 | 2.5% |
| Biopsy | 2 | 2.5% |
| Lobectomy+Double wedge resection | 1 | 1.2% |
| Double Lobectomy | 1 | 1.2% |
| Enucleation | 1 | 1.2% |
| n.a. | 1 | 1.2% |
| Total | 81 | 100.0% |
Out of 81 patients, 32 (40%) received chemotherapy, Other 47 patients did not receive any oncologic treatment. Survival after surgery has been studied with Kaplan -Meyer Cox – Regression method.
Results
In our series, there was no intro/perioperative mortality or post-operative morbidity. Chest tubes have been removed after a mean time of 4.2 days (2 days to 8 days).
Median overall survival was 5.9 years (2.6 years to 9.1 years).
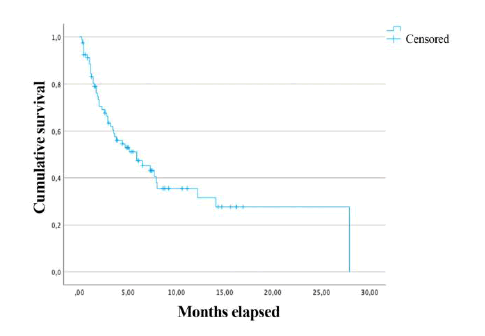
The overall survival of all patients has been 82.7% at 1 year, (67 patients), 54.3% at 3 years (44 patients), and 37% at 5 years (30 patients) (Figure 1).
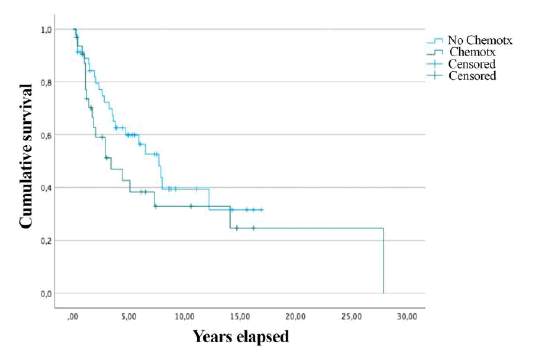
Overall survival of 32 patients who received chemotherapy was compared to that of 49 patients who didn’t. In the first group (CT) overall survival was 3.4 years (0.6-6.1), while in the second group (no CT) it resulted in 7.7 years (4.9-10.4). However, the difference in overall survival has not revealed statistical significance (p=0.18) (Figure 2)
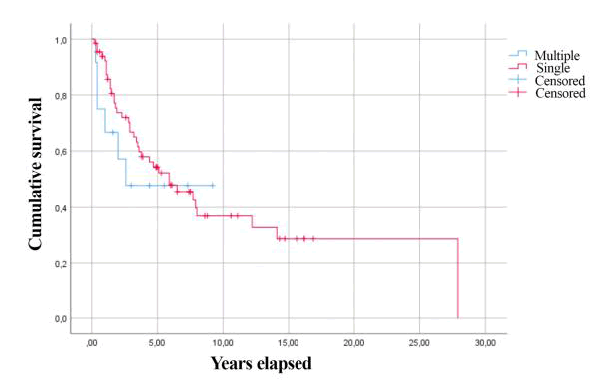
The mean survival of patients who submitted lung surgery for single and multiple metastases was respectively 5.9 and 2.6 years; no statistical significance was evidenced (p = 0.52) (Figure 3).
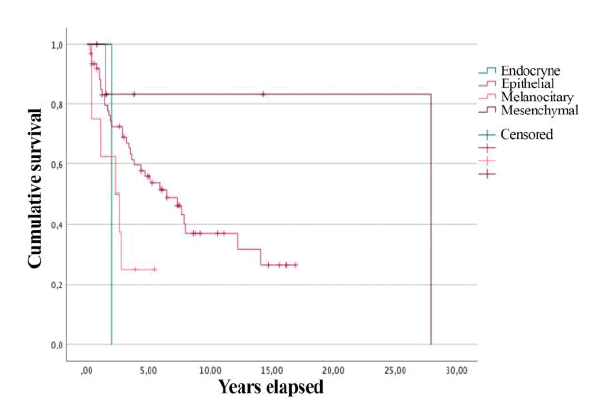
At last overall survival was calculated after the division of patients into 4 groups, based on the histogenesis of primitive neoplasm. In the “epithelial” group overall survival was 6.5 years (3.2-9.7), in the “melanocitary” group 2.3 years (0.2-4.3), in the “mesenchymal” group 23 years (12-34) and the “endocrine” group 2 years (1.2-3.1). Comparison of overall survival curves presented statistical significance (p = 0.05) (Figure 4).
Discussions
Our experience in the surgical treatment of lung metastases results in the main goals reported in the literature [1,25-28]. According to some of the most important Authors, overall survival at 5 years was 37% (Vogt-Moykopf 33% in 1994, Gregory A. Master 35% in 1995 and U. Pastorino 36% in 1997) [1]. Oncological criteria we adopted for patient selection were: (I) completely cured or curable primary cancer, (II) lung as the only site of metastases/ lesions in other singular sites but lung, suitable for radical treatment, (III) lesions that technically can be completely removed, (IV) disease-free interval longer than 24 months and (V) clinical conditions and lung function permissive for intervention [29]. All resections were R0. We did not focus on systematic lymphadenectomy since there is no evidence of a clear benefit as survival is concerned [30-32]. The absence of intra/perioperative mortality associated with a short time of chest tube maintenance confirms a low surgical risk and the feasibility of iterative lung surgery [33,34]. We found a quite better overall survival for mesenchymal neoplasms while the poorest was for endocrine ones. Differently from other experiences in our series we didn’t find a better overall survival at 5 years for patients who received chemo versus the ones who didn’t. On the contrary, even if a statistical significance was not reached, we observed a better overall survival for patients who didn’t receive chemo versus the ones who did. This result can be explained by considering the heterogeneous histotype cases and the limited number of patients in our series [1,35,36]. Probably because of the same reason the difference in survival for patients with single metastasis vs patients with multiple ones resulted not have statistical value even if, by other authors patients with single metastasis had better overall survival at 5 years [1,37,38]. In our series, good overall survival at 5 years, absence of perioperative mortality/morbidity, and short maintenance of chest tube are reasonably due to:
•Prevalence of single metastases (70 patients) vs multiple lesions (11 patients).
• Optimal selection of patients.
Patients have to be studied with a CT scan of the chest, brain, and abdomen, bronchoscopy, PET, and with accurate functional evaluation [39,40].
However, even if repeated pulmonary metastasectomy is justified, careful considerations should be taken before a potential second or third metastasectomy [41,42]. If patients present multiple poor prognostic factors and/or a short disease-free interval, it should be better to observe the patient for a certain period before deciding about an indication for further pulmonary resection.
Conclusion
In conclusion surgery for lung metastasis when feasible requires an accurate selection of patients and should be done within a multidisciplinary assessment, with particular care to oncologic and histopathologic issues. It is recommended a complete exploration of the lung parenchyma to perform a radical surgery, avoiding if possible removing healthy tissue, and also for further potential iterative surgery.
After metastasectomy follow-up should be very careful because of the risk of recurrence; in this case, the patient has to be evaluated for surgery at first since iterative surgery of lung metastases represents a favorable prognostic factor. Surgery for lung metastases nowadays has a diagnostic role, reveals useful for radiation, and especially represents a valid therapy as an “oncologic clearance of organ”. To reach an oncologic radical clearance manual palpation remains safe support for the surgeon, particularly in cases where there is a clinical suspicion of misdiagnosed lesions, even if some Authors do not agree. They found no difference in survival after lung metastasectomy for what disease-free interval and recurrence is concerned both for VATS both for an open surgical approach.
Declarations
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Pastorino, Ugo, et al. "Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases." The Journal of thoracic and cardiovascular surgery, Vol. 113, No. 1, 1997, pp. 37-49.
Google Scholar Crossref - Yıldız, Oya, et al. "Survival outcome of pulmonary metastasectomy among the patients with colorectal cancers." Revista da Associação Médica Brasileira, Vol. 67, 2021, pp. 1015-20.
Google Scholar Crossref - Lanza, Louis A., et al. "Response to chemotherapy does not predict survival after resection of sarcomatous pulmonary metastases." The Annals of thoracic surgery, Vol. 51, No. 2, 1991, pp. 219-24.
Google Scholar Crossref - Robert, John H., et al. "Factors influencing long-term survival after lung metastasectomy." The Annals of thoracic surgery, Vol. 63, No. 3, 1997, pp. 777-84.
Google Scholar Crossref - Van Geel, Albert N., et al. "Surgical treatment of lung metastases: the European Organization for Research and Treatment of Cancer‐Soft Tissue and Bone Sarcoma Group study of 255 patients." Cancer: Interdisciplinary International Journal of the American Cancer Society, Vol. 77, No. 4, 1996, pp. 675-82.
Google Scholar Crossref - Mountain, Clifton F., Marion J. McMurtrey, and Kay E. Hermes. "Surgery for pulmonary metastasis: a 20-year experience." The Annals of thoracic surgery, Vol. 38, No. 4, 1984, pp. 323-30.
Google Scholar Crossref - Mountain, Clifton F., Marion J. McMurtrey, and Kay E. Hermes. "Surgery for pulmonary metastasis: a 20-year experience." The Annals of thoracic surgery, Vol. 38, No. 4, 1984, pp. 323-30.
Google Scholar Crossref - Girard, Phillippe, et al. "Surgery for pulmonary metastases. Who are the 10‐year sruviors?." Cancer, Vol. 74, No. 10, 1994, pp. 2791-97.
Google Scholar Crossref - Grunenwald, Dominique, et al. "Completion pneumonectomy for lung metastases: is it justified?." European journal of cardio-thoracic surgery, Vol. 12, No. 5, 1997, pp. 694-97.
Google Scholar Crossref - Ueda, Takafumi, et al. "Aggressive pulmonary metastasectomy for soft tissue sarcomas." Cancer, Vol. 72, No. 6, 1993, pp. 1919-25.
Google Scholar Crossref - Todd, Thomas R. "The surgical treatment of pulmonary metastases." Chest, Vol. 112, No. 4, 1997, pp. 287-90.
Google Scholar Crossref - Pastorino, Ugo, et al. "The contribution of salvage surgery to the management of childhood osteosarcoma." Journal of clinical oncology, Vol. 9, No. 8, 1991, pp. 1357-62.
Google Scholar - Putnam JR, Joe B., and Jack A. Roth. "Prognostic indicators in patients with pulmonary metastases." Seminars in Surgical Oncology, Vol. 6. No. 5, 1990 pp. 291-96.
Google Scholar Crossref - Marincola, Francesco M., and James BD Mark. "Selection factors resulting in improved survival after surgical resection of tumors metastatic to the lungs." Archives of Surgery, Vol. 125, No. 10, 1990, pp. 1387-93.
Google Scholar Crossref - Treasure, Tom, and Fergus Macbeth. "Pulmonary metastasectomy: limits to credibility." Journal of Thoracic Disease, Vol. 13, No. 4, 2021, pp. 2603-10.
Google Scholar Crossref - Bains, Manjit S., et al. "The clamshell incision: an improved approach to bilateral pulmonary and mediastinal tumor." The Annals of thoracic surgery, Vol. 58, No. 1, 1994, pp. 30-33.
Google Scholar Crossref - Dowling, Robert D., Peter F. Ferson, and Rodney J. Landreneau. "Thoracoscopic resection of pulmonary metastases." Chest, Vol. 102, No. 5, 1992, pp. 1450-54.
Google Scholar Crossref - Roth, Jack A., et al. "Comparison of median sternotomy and thoracotomy for resection of pulmonary metastases in patients with adult soft-tissue sarcomas." The Annals of thoracic surgery, Vol. 42, No. 2, 1986, pp. 134-38.
Google Scholar Crossref - Eckardt, Jens, and Peter B. Licht. "Thoracoscopic versus open pulmonary metastasectomy: a prospective, sequentially controlled study." Chest, Vol. 142, No. 6, 2012, pp. 1598-02.
Google Scholar Crossref - Landreneau, Rodney J., et al. "Therapeutic video-assisted thoracoscopic surgical resection of colorectal pulmonary metastases." European journal of cardio-thoracic surgery 18.6 (2000): 671-677.
Google Scholar Crossref - Girard, Philippe, et al. "Surgical resection of pulmonary metastases. Up to what number?." American journal of respiratory and critical care medicine, Vol. 149, No. 2, 1994, pp. 469-76.
Google Scholar Crossref - Putnam, Joe B., et al. "Extended resection of pulmonary metastases: is the risk justified?." The Annals of thoracic surgery, Vol. 55, No. 6, 1993, pp. 1440-46.
Google Scholar Crossref - Takita, Hiroshi, et al. "The surgical management of multiple lung metastases." The Annals of Thoracic Surgery, Vol. 24, No. 4, 1977, pp. 359-64.
Google Scholar Crossref - Shimizu, Nobuyoshi, et al. "Transsternal thoracotomy for bilateral pulmonary metastasis." Journal of surgical oncology, Vol. 50, No. 2, 1992, pp. 105-09.
Google Scholar Crossref - Todd, Thomas R. "Pulmonary metastectomy: current indications for removing lung metastases." Chest, Vol. 103, No. 4, 1993, pp. 401-03.
Google Scholar Crossref - Groeger, A. M., et al. "Survival after surgical treatment of recurrent pulmonary metastases." European journal of cardio-thoracic surgery, Vol. 12, No. 5, 1997, pp. 703-05.
Google Scholar Crossref - Matthay, Richard A., and Alejandro C. Arroliga. "Resedion of Pulmonary Metastases." American Review of Respiratory Disease, Vol. 148, 1993, pp. 1691-96.
Google Scholar Crossref - Vogt-Moykopf, I., et al. "Surgery for pulmonary metastases. The Heidelberg experience." Chest Surgery Clinics of North America, Vol. 4, No. 1, 1994, pp. 85-12.
Google Scholar - Milano, S., et al. "Il trattamento chirurgico delle metastasi polmonari." Quaderni di medicina e chirurgia, Vol. 13, 1997, pp. 31-35.
Google Scholar - Yang YH, et al. "Effects of mediastinal lymph node dissection in colorectal cancer-related pulmonary metastasectomy." Thorac Cancer, Vol. 12, No. 23, 2021, pp. 3248-54.
Crossref - Londero F, et al. "Lymphadenectomy during pulmonary metastasectomy: Impact on survival and recurrence." Journal of Surgical Oncology, Vol. 120, No. 4, 2019, pp. 768-78.
Crossref - Welter S, Gupta V, and Kyritsis I. "Lymphadenectomy in pulmonary metastasectomy." Journal of Thoracic Disease, Vol. 13, No. 4, 2021, pp. 2611-17.
Crossref - Friedrich Schildberg W., Guenter M. Stefan-Piltz and Hans G. Koebe, et al. "Surgical treatment of tumor metastases: general considerations and results." Surgery Today, Vol. 25, No. 1, 1995, pp. 1-10.
- Liebl LS., et al. "Value of repeat resection for survival in pulmonary metastases from soft tissue sarcoma." Anticancer Research, Vol. 27, No. 4C, 2007, pp. 2897-02.
- Robert, John H., et al. "Factors influencing long-term survival after lung metastasectomy." The Annals of thoracic surgery, Vol. 63, No. 3, 1997, pp. 777-84.
Google Scholar Crossref - Saeter, G., et al. "Ifosfamide and continuous infusion etoposide in advanced adult soft tissue sarcoma. A Scandinavian Sarcoma Group Phase II Study." European Journal of Cancer, Vol. 33, No. 10, 1997, pp. 1551-58.
Google Scholar Crossref - Girard, Philippe, et al. "Should the number of pulmonary metastases influence the surgical decision?." European Journal of Cardio-thoracic Surgery: Official Journal of the European Association for Cardio-thoracic Surgery, Vol. 12, No. 3, 1997, pp. 385-91.
Google Scholar Crossref - McIntosh R, and Thatcher N. "Management of the solitary metastasis." Thorax. Vol. 45, No. 12. 1990, pp. 909-11.
Crossref - Pastorino U, et al. "Fluorodeoxyglucose positron emission tomography improves preoperative staging of resectable lung metastasis." Journal of Thoracic and Cardiovascular Surgery, Vol. 126, No. 6, 2003, pp. 1906-10.
Crossref - Forster C, et al. "Is repeated pulmonary metastasectomy justified?." Clinical & Experimental Metastasis, Vol. 37, No. 6, 2020, pp. 675-82.
- Yang KM,. et al. "Benefits of repeated resections for liver and lung metastases from colorectal cancer." Asian Journal of Surgery, Vol, 43, No. 1, 2020, pp. 102-09.
Crossref - Kanzaki R., et al. "Preoperative evaluation and indications for pulmonary metastasectomy." Journal of Thoracic Disease, Vol. 13, No. 4, 2021, pp. 2590-02.
Crossref