Research - International Journal of Medical Research & Health Sciences ( 2023) Volume 12, Issue 8
Molecular Docking Study of Selected Bio-Active Compounds on Alzheimer's Disease using BACE-1 (Pdb ID: 5QCU) as Target Protein
Ebube S Chidera1, Ashish B Gulwe2* and Awote O Kayode12Swami Ramanand Teerth Marathwada University, Sub Campus, School of Technology Latur, India
Ashish B Gulwe, Swami Ramanand Teerth Marathwada University, Sub Campus, School of Technology Latur, India, Email: ashishgulwe@gmail.com
Received: 13-Jul-2023, Manuscript No. ijmrhs-23-105961; Editor assigned: 18-Jul-2023, Pre QC No. ijmrhs-23-105961(PQ); Reviewed: 15-Aug-2023, QC No. ijmrhs-23-105961(Q); Revised: 25-Aug-2023, Manuscript No. ijmrhs-23-105961(R); Published: 30-Aug-2023
Abstract
Alzheimer’s Disease (AD) is a complex neurodegenerative disease that is characterized by the accumulation of amyloid-beta (Aβ) peptides in the brain. It is the most common type of dementia which begins with mild memory loss and leads to severe decline in one’s ability to hold adequate conversation and response with the environment. β-secretase-1(BACE-1) is a key enzyme involved in the production of Aβ peptides, making it an attractive target for drug discovery in AD treatment. Herein, this study aimed to investigate the anti-alzheimer’s potential of selected bioactive compounds against BACE-1 protein. Molecular docking was employed using Pyrx and Biovia discovery studio software to predict potential selected bioactive antagonists and non-covalent interactions between the selected ligands, standard drugs and the target protein. BACE-1 target protein was docked with ligands namely; Tacrine, Harmine, Coumarin, Berberine, Indole, Resveratrol, Huperzine, 3-chloro-R(2),C(6)-bis(4-fluorophenyl)-3- methylpipiridin-4-one (CFMP), and the standard alzheimer’s drugs namely; Donepezil and Galantamine after which the ligand with the best binding affinity was determined. The docking result from this study revealed Resveratrol as the ligand with the best binding affinity when docked with the selected Alzheimer’s target proteins.
Keywords
Alzheimer’s Disease (AD), Bioactive compounds, Molecular docking, Galantamine, Resveratrol, Donepezil, Pyrx, Discovery studio, Binding affinity
Introduction
According to the Alzheimer’s disease International, it has been estimated that a total of 55 million people is currently suffering from dementia and the estimated number is projected to increase twice as much until the year 2050 with the greatest increase in low-income countries and middle-income countries. Every three (3) seconds, a new case of dementia is reported. According to World Alzheimer Report 2015, if ‘dementia’ was a country, it would be the world’s 18th largest economy.
The term “Dementia” is used to describe a variety of brain illnesses that impact memory, reasoning, behaviour and emotion. There are various other causes of dementia namely vascular disease, dementia with lewy bodies, fronto-temporal dementia and Alzheimer’s diseases. Alzheimer’s Disease (AD) is a common type or cause of dementia which affects 50% to 60% of people with dementia [1].
Alzheimer’s Disease (AD) has a progressive nature and it eventually leads to a severe cognitive decline. It has grown to be a significant issue, especially in developed nations given to an aging population with a high standard of living. It primarily affects older people (people older than 65 years old), although it can also be observed in young people.
In the brains of patients with Alzheimer’s, the size of the ventricles increases whilst the size of the cerebral cortex and hippocampus shrinks. Memory functions such as episodic memory and spatial memory become impaired when the hippocampus' size is decreased. This damage between neurons however leads to communication defects in planning, judgment, and short-term memory. This reduction causes impairment of the synapses, neuron ends, and further cell loss [2].
Although, the actual pathophysiology of AD has not been fully elucidated due to its complexity, several mechanisms have been suggested to explain its pathophysiology. These mechanisms include; loss of cholinergic neurotransmission (that is, neurotransmitters such as acetylcholine, dopamine, and serotonin), deposition of βamyloid (Aβ) plaques, accumulation of hyper-phosphorylated tau-protein, and increased oxidative stress. Various strategies or hypothesis has also been proposed to deal with the suggested pathophysiological effects. The proposed strategies are; Cholinergic hypothesis and Amyloid hypothesis.
According to the Amyloid hypothesis, the deposition of extracellular aggregates of Aβ-peptide initiates the pathogenic cascade that leads to neuronal cell death associated with Alzheimer’s (AD). BACE-1 is highly expressed in the brain and it is a key enzyme in the amyloidogenic signaling pathway with attractive therapeutic targets in AD, including inhibition of Aβ generation and β-secretase. The enzyme plays a key role in the brain's production of the neurotoxic β-amyloid (Aβ) peptides that cause Alzheimer’s Disease (AD) [3].
According to the Cholinergic hypothesis, a decline of cognitive function is related to the decreased concentration of pre-synaptic neurotransmitter Acetylcholine (ACh) in the brain. Acetylcholine (ACh) has been found to have a strong correlation with memory performance, including memory encoding, consolidation storage as well as serving a potential role in Aβ-aggregation in Alzheimer patients. The following Acetylcholinesterase inhibitors (AChEIs); Donepezil, Galantamine, and Rivastigmine are recognized for the treatment of Alzheimer’s however these drugs can only help improve cognitive symptoms of Alzheimer’s for a certain period of time, they cannot modify the course of the disease. Hence the need for this research which aims to propose active compounds/drugs which have a stronger efficacy and energy in modifying the disease [4].
Materials and Method
Materials
The web resources used were Pubchem, Pubmed, RCSB Protein Data Bank, Admet SAR, Swissadme, Novoprolabs whilst the software used for the docking experiment were Pyrx, Biovia Discovery Studio and Autodock Vina Wizard.
Methodology
Drug likeness and pharmacokinetic prediction: SwissADME and admetSAR were used to predict the druglikeness, pharmacokinetic and blood-brain barrier parameters and also check if the docked ligands obey the Lipinski rule of five [5].
Ligand modelling: The 3D crystal structures of the ligands were retrieved from Pubchem database in .sdf format and converted into .pdb using Biovia Discovery Studios 2021. The canonical SMILES of the ligands were also retrieved from the Pubchem database and the SMILES of the ligands were converted into .mol 3D structure using Novoprolabs.
The canonical SMILES explain the molecular structure as a graph with optional chiral indications. The energy of ligand molecules was minimized using mmff94 force field and conjugate gradients as an optimization algorithm. Energy minimization is an important step in the preparation of a ligand to abolish clashes within the atoms of a ligand molecule and produce a reasonable starting pose. Using Pyrx, the energy minimized ligand molecules were then converted into AutoDock ligand (that is, its .pdbqt format) [6].
Protein (target) preparation: The 3D crystal structure of the protein target: BACE-1 (PDB ID: 5QCU, 1.95 resolution) was retrieved from protein data bank. The 3D structure of the protein was modelled based on BACE-1 homosapiens with high resolution, sequence identity, domain coverage and E-value after blasting
Biovia discovery studio 2021 was used to remove water molecules and heteroatoms and also to add polar hydrogen. Water molecules and other heteroatoms were removed to clear the binding pocket and make computations easier so that ligand can create satisfying interactions with the protein. The chain was selected based on their completeness of amino acid residue and presence of the active site. This modified protein was then saved in .pdb format [7].
Molecular docking: For molecular docking studies, AutoDock Vina was employed and gradient-based conformational search was used in Auto Vina docking. Donepezil and Galantamine (both of which are the standard medications for Alzheimer) as well as the tested ligands of the target protein were used. The study was based on binding free energies and Root Mean Square Deviation (RMSD) values, and the ligand molecules were then sorted in the order of increasing docking energies. Each ligand underwent a docking experiment consisting of 100 stimulations of the lowest energy that each binding energy can represent [8].
Results
Table 1 shows the ligands, its obedience to the Lipinski rule of five and its respective blood-brain barrier. The table shows that all the lead molecules/ligands obey the Lipinski rule of five (RO5) with tacrine and galantamine having the highest Blood-Brain Barrier (BBB) probability whilst Resveratrol has the lowest Blood-Brain Barrier (BBB) probability.
| Serial No. |
Lead molecule/Ligand | Obedience | Blood-Brain Barrier | |
|---|---|---|---|---|
| Value | Probability | |||
| 1 | Tacrine | Yes | + | 1 |
| 2 | Coumarin | Yes | - | 0.725 |
| 3 | Harmine | Yes | + | 0.7879 |
| 4 | Berberine | Yes | + | 0.575 |
| 5 | Indole | Yes | + | 0.975 |
| 6 | Galantamine* | Yes | + | 1 |
| 7 | Resveratrol | Yes | - | 0.65 |
| 8 | Donepezil* | Yes | + | 0.925 |
| 9 | 3-chloro-R(2),C(6)-bis(4fluorophenyl)-3-methylpipiridin-4-one (CFMP) | Yes | + | 0.925 |
| 10 | Huperzine | Yes | + | 0.8 |
Table 2 shows the binding affinity of all ligands against the protein (BACE-1). Binding affinity is used to enumerate the binding strength of ligands to selected target protein or to proteins. The results revealed that Resveratrol has the strongest binding affinity. Herein, Harmine has the lowest binding affinity of -5.5 Kcal/Mol and Resveratrol has the highest binding affinity of -7.9 Kcal/Mol.
| Serial No. | Ligands | Binding Affinity |
|---|---|---|
| 1 | Tacrine | -5.6 |
| 2 | Harmine | -5.4 |
| 3 | Coumarin | -6.1 |
| 4 | Berberine | -7.4 |
| 5 | Indole | -5.5 |
| 6 | Galantamine* | -7.7 |
| 7 | Resveratrol | -7.9 |
| 8 | Donepezil* | -6.6 |
| 9 | 3-chloro-R(2), C(6)-bis(4-fluorophenyl)-3-methylpipiridin-4-one (CFMP) | -7.7 |
| 10 | Huperzine | -6.4 |
|
Note: Ligands with asterisks (*) are the standard drugs used to manage Alzheimer |
||
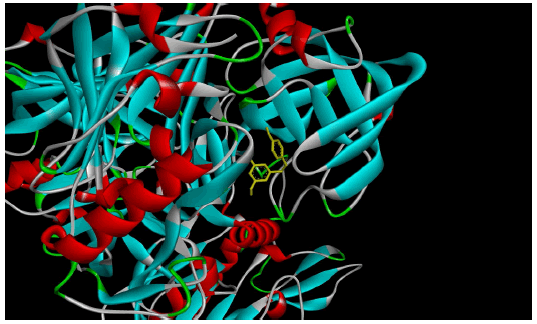
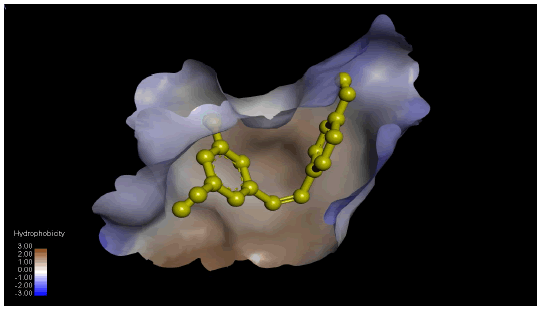
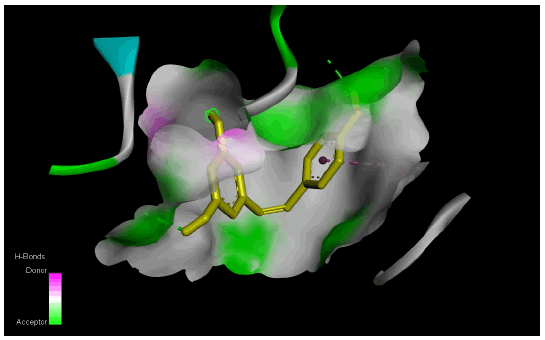
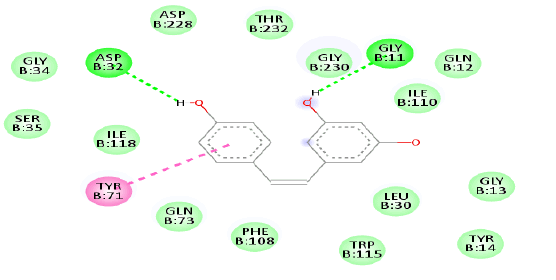
Figure 1(a) shows the 3D crystal structure of protein-ligand (BACE-1-Resveratrol) complex interaction. The hydrophobicity and hydrogen bond interaction of BACE-1 docked with the ligand with the best binding affinity (Resveratrol) is shown in Figure 1(b) and Figure 1(c) respectively. Figure 1(d) shows the 2D interaction of the ligand (Resveratrol) with the best binding affinity.
Discussion
Molecular docking is an in-silico modeling technique used to study the interaction between protein targets and small ligands (compounds). The molecular docking analysis provided valuable insights into the binding modes and affinities of the selected bio-active compounds towards BACE-1. The docking results of some of these bioactive compounds such as; Resveratrol, Galantamine, Huperzine, Berberine, Donepezil and 3-chloro-R(2),C(6)-bis(4- fluorophenyl)-3-methylpipiridin-4-one (CFMP) had strong affinity to the target protein BACE-1 (PDB ID: 5QCU), indicating its potential to interact with the active sites of the protein and modulate its enzymatic activity. The favourable interactions observed included hydrogen bonding, hydrophobic contacts, and π-π stacking interactions, which are critical for stabilizing the ligand-protein complex.
The identification of key residues involved in the binding interactions between the compounds and BACE-1 is of significant importance. By analyzing the docked complexes, the specific amino acid residue within the active sites of BACE-1 that contributes to the binding affinity of the compounds was determined. These residues may serve as crucial drug targets for future drug design and optimization. Additionally, the structural features responsible for the observed binding affinity such as the presence of specific functional groups or aromatic moieties in the compounds, can guide the development of more potent and selective BACE-1 inhibitors. The docking results of standard drugs which are available in the markets like Galantamine and Donepezil shows the Binding affinity as -7.7 Kcal/Mol and -6.6 Kcal/Mol with the Target protein BACE-1. The binding affinity value of the standard drugs were taken into account as reference values in comparison to the binding affinities with the natural bioactive compounds binding affinities and it was discovered that resveratrol had the best binding affinity followed by berberine.
The molecular binding images and hydrophobic interaction of 2D plot also supports the significant binding free energy (Gibbs free energy) of these bioactive compounds.
Conclusion
This study compared the binding affinity of all the selected bio-active compounds and the known standard drugs to determine which has a stronger binding affinity and a possible anti-alzheimer potential. Hence, it can be concluded that these bioactive compounds can be replaced with the standard drugs available in the markets for the Alzheimer’s disease treatment although further investigations or studies involving clinical trials and lab testing can be initiated.
Acknowledgment
We thank the Swami Ramanad Teerth Marathwada University Sub Campus Latur for providing computational lab resources for conducting the experiments. We are also very grateful to Mr. Awote Olasunkanmi Kayode of the Lagos State University for collaborative research work. We hope we will produce excellent research on different human diseases and their therapeutic treatments
Declarations
Ethical Considerations
All related research's ethical principles are considered in this article.
Conflict of Interest
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
References
- Arulraj, Ramalingam. "Hirshfeld surface analysis, interaction energy calculation and spectroscopical study of 3-chloro-3-methyl-r (2), c (6)-bis (p-tolyl) piperidin-4-one using DFT approaches." Journal of Molecular Structure, Vol. 1248, 2022.
Google Scholar Crossref - Dawood, Rafid S., and Sudad A. Dayl. "Synthesis, identification and molecular docking studies of N-functionalized piperidine derivatives linked to 1, 2, 3-triazole ring." Synthetic Communications, Vol. 50, No. 16, 2020, pp. 2422-31.
Google Scholar Crossref - Ramalingam, Arulraj, et al. "Synthesis, crystal structure, DFT calculations and Hirshfeld surface analysis of 3-chloro-2, 6-bis (4-chlorophenyl)-3-methylpiperidin-4-one." Journal of Chemical Crystallography, Vol. 51, 2021, pp. 273-87.
Google Scholar Crossref - Sagaama, Abir, and Noureddine Issaoui. "Design, molecular docking analysis of an anti-inflammatory drug, computational analysis and intermolecular interactions energy studies of 1-benzothiophene-2-carboxylic acid." Computational biology and chemistry, Vol. 88, 2020.
Google Scholar Crossref - Romani, Davide, et al. "Properties and reactivities of niclosamide in different media, a potential antiviral to treatment of COVID-19 by using DFT calculations and molecular docking." Biointerface Research in Applied Chemistry, Vol. 10, No. 6, 2020, pp. 7295-328.
Google Scholar Crossref - Ullah, Hayat, et al. "New oxadiazole bearing thiosemicarbazide analogues: Synthesis, anti-alzheimer inhibitory potential and their molecular docking study." Chemical Data Collections, Vol. 41, 2022.
Google Scholar Crossref - Gulwe, Ashish B., et al. "MOLECULAR DOCKING STUDY OF SELECTED BIO-ACTIVE COMPOUNDS IN ALZHEIMER’S DISEASE USING BACE-1 (PDB ID: 5QCU) AS TARGET PROTEIN." Journal of Population Therapeutics and Clinical Pharmacology, Vol. 30, No. 3, 2023, pp. 787-93.
Google Scholar Crossref - Chidambaram, Kumarappan. "Identification of BACE-1 Inhibitors against Alzheimer’s Disease through E-Pharmacophore-Based Virtual Screening and Molecular Dynamics Simulation Studies: An Insilco Approach." Life, Vol. 13, No. 4, 2023, p. 952.
Google Scholar Crossref