Research - International Journal of Medical Research & Health Sciences ( 2021) Volume 10, Issue 6
Pattern of Adverse Drug Reactions (ADRs) Reported with Neuropsychiatric Drugs in a Teaching Hospital of North Karnataka: An Observational Study
Anant Khot1*, Shivakumar P Chaukimath2 and Akram A Naikwadi32Department of Psychiatry, BLDE(DU)’s Shri BM Patil Medical College and RC, Vijayapura, India
3Department of Pharmacology, BLDE(DU)’s Shri BM Patil Medical College and RC, Vijayapura, India
Anant Khot, Department of Pharmacology, AIIMS, Nagpur, India, Email: anantkhot04@gmail.com
Received: 19-May-2021 Accepted Date: Jun 23, 2021 ; Published: 30-Jun-2021
Abstract
Purpose: Adverse Drug Reactions (ADRs) to neuropsychiatric drugs are common and can lead to noncompliance. As patients with neuro or psychiatric illness need long-term therapy with psychotropic medications and the pharmaceutical companies utilize this opportunity to influence the prescribing habits of the treating physician. If the patients receive polypharmacy, they will be predisposed to an array of side effects. Hence, the treating physician needs to consult the safety profile and cost of the therapy before prescribing any psychotropic medications. If the patient discontinues the therapy due to adverse effects, it can lead to the recurrence of the symptoms or disease relapse. Over the last few years, atypical (Second generation) antipsychotics have been increasingly used to treat schizophrenia and other related neuropsychiatric disorders. Along with that, the safety profile of few newer atypical antipsychotic agents and other drugs used in neuropsychiatric illnesses has not been elucidated completely. To determine the pattern of ADR’s caused by these medications and the methods used to prevent an ADR related morbidity, this study has been undertaken. Methods: All the spontaneously reported ADR’s between Aug 2015 to Sep 2017, to an ADR monitoring center in Vijayapura, after the administration of drugs effective in the treatment of neuropsychiatric illnesses, were collected and entered into the suspected ADR form designed by the IPC (Indian Pharmacopoeia Commission). The data gathered was assessed for causality by using, World Health Organization-Uppsala Monitoring Centre (WHOUMC) causality assessment system. Similarly, the severity and preventability were assessed by using standard prevalidated scales. Results: A total of 177 ADRs were identified from 104 patients, and the vast majority of those patients who had an ADR belong to the age group of 21-40 years. Among the total, 84 (80.8%) patients had to Type A ADRs. Nausea being the commonest ADR reported and the System Organ Class (SOC) which got affected was neurological disorders as coded in the MedDRA. Psycholeptics were predominantly involved in the causation of ADRs. The ADRs were mostly mild to moderate in severity and only 2 patients had severe cutaneous reactions with Antiepileptics. 48.07% of the ADR’s were preventable according to the Schumock criteria. Conclusion: The present study adds to the existing information on the safety of the medications used in the treatment of neuropsychiatric illnesses especially in South Indian patients. Still incomplete because of underreporting by the Health care providers. Hence, further research by targeted pharmacovigilance activity or active surveillance is needed to strengthen the database.
Keywords
Pharmacovigilance, Psychotropic drugs, Adverse drug reaction
Introduction
Adverse drug reaction can be defined as, “An appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen or withdrawal of the product” [1].
A meta-analysis of 39 epidemiological studies by Lazarou, et al. found that ADRs ranked fourth and sixth leading causes of death in the USA. The median incidence of ADRs that lead to hospitalization and that developed during hospitalization were 2.85% and 6.34% respectively. The fatal ADR incidence was 0.08% (95% CI: 0%-0.15%) [2].
Analysis of 15 epidemiological studies on psychiatric morbidity in India was carried out and according to them, the national prevalence rate for ‘all mental disorders’ is 73 per 1000 population [3]. Patients with a neuropsychiatric illness need long-term therapy with psychotropic drugs, which predisposes them to an array of ADRs. The common adverse effects associated with psychotropic drugs are weight gain, somnolence, tremors, and tardive dyskinesia. Identification of these adverse effects requires careful consideration of other neuropsychiatric and medical disorders that may mimic antipsychotic-related side-effects. These adverse effects tend to complicate the mental and physical well-being of the patient and thus lead to patient non-adherence to therapy [4]. Effective management of these adverse effects has the potential to improve patient’s compliance, quality of life & possibly the prognosis and outcome [5]. Over the last few years, second-generation (atypical) antipsychotics have been increasingly used in the treatment of schizophrenia and other related neuropsychiatric disorders. Manufacturers have also initiated campaigns to promote their use for other off-label indications. The effectiveness and safety profile of few newer atypical antipsychotic agents have not been elucidated completely. Hence, to know the pattern of ADRs and the methods used to prevent ADRrelated morbidity in this part of the country, the above study has been carried out.
Materials and Methods
Source of Data
ADRs were reported from the Department of Psychiatry, Medicine, Pediatrics, and the Dermatology Department of Shri. B. M. Patil Medical College hospital, following the administration of drugs effective in the treatment of neuropsychiatric illnesses.
Duration of the Study
From August 2015 to September 2017.
Method of Data Collection
Details of the spontaneously reported ADRs by the health care professionals through telephone were collected in person from the ward and entered into the suspected ADR form designed by the Indian Pharmacopoeia Commission (IPC). The remaining Individual Case Safety Reports (ICSRs) were sent through WhatsApp with relevant information, images and videos. That information was also entered into the suspected ADR reporting form.
The information gathered was assessed for causality by using World Health Organization-Uppsala Monitoring Centre (WHO-UMC) causality assessment system, for preventability by using Schumock-Thornton criteria and for severity by using the criterion developed by Hartwig et al. [6-8]. After the assessment, the ICSRs were entered into the Vigiflow software, and the Portable Document Format (PDF) generated after uploading it was used for data entry and analysis. Ethical clearance was obtained from the Institutional Ethics Committee of BLDE (Deemed to be University).
Statistics
Data were analyzed by using SPSS 23, IBM obtained from SPSS South Asia Private Limited, India. Microsoft excel 2016 was used to design graphs and pie charts.
Results
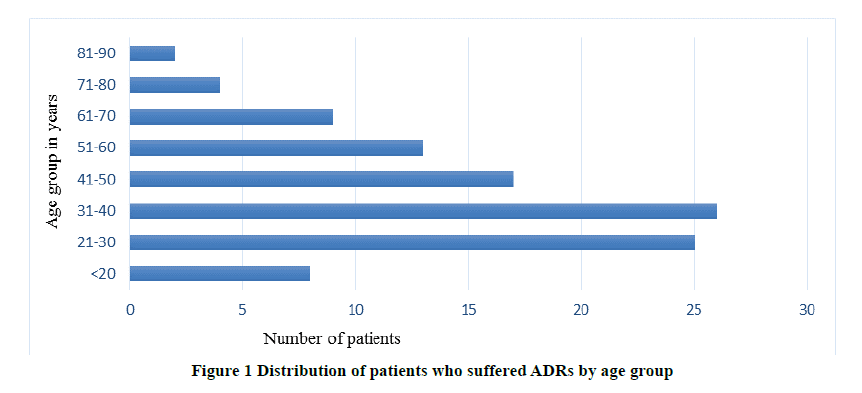
A total of 177 ADRs were identified from 104 patients. Figure 1 shows the distribution of patients by age group, who suffered from ADRs. As we can notice from Figure 1, the majority of the patients who had an ADR belong to the age group of 21-40 years. Among 104 patients, 52 were males and 52 were females. 84 (80.8%) patients had Type A, 14 (13.5%) had Type B and 6 (5.8%) had Type C ADRs, according to the extended Rawlins-Thompson classification. The neuropsychiatric conditions were diagnosed and entered into the Vigiflow by using the International Classification of Diseases-10 (ICD-10) codes.
According to Table 1, the patients with schizophrenia and bipolar affective disorders had the majority of the ADRs following Pharmacotherapy. Table 2 shows the System Organ Class (SOC) involved as coded under the Medical Dictionary for Regulatory Activities (MedDRA) with representative ADRs mentioned on the right-side column [9].
Table 1 Shows the spectrum of diseases for which pharmacotherapy was given
| ICD class | Frequency | Percentage |
|---|---|---|
| Acute and transient psychotic disorders | 1 | 0.96 |
| Other anxiety disorders | 5 | 4.8 |
| Bipolar affective disorder | 19 | 18.26 |
| Depressive episode | 18 | 17.3 |
| Dementia in Alzheimer's disease | 2 | 1.92 |
| Delusional disorders | 4 | 3.84 |
| Epilepsy | 10 | 9.61 |
| Febrile convulsions | 1 | 0.96 |
| Hepatic failure (Alcoholic) | 1 | 0.96 |
| Insomnias | 1 | 0.96 |
| Manic episode | 3 | 2.88 |
| Migraine | 4 | 3.84 |
| Obsessive-compulsive disorder | 1 | 0.96 |
| Panic disorder | 5 | 4.8 |
| Schizophrenia | 20 | 19.23 |
| Schizoaffective disorder | 1 | 0.96 |
| Somatization disorder | 1 | 0.96 |
| Specific personality disorders | 2 | 1.92 |
| Treatment resistant depression | 1 | 0.96 |
| Unspecified nonorganic psychosis | 2 | 1.92 |
| Vascular headache | 1 | 0.96 |
| Mental and behavioral disorders due to use of alcohol | 1 | 0.96 |
| Total | 104 | 100 |
Among 104 patients who had ADRs, the majority of them (56) belong to the SOC of neurological disorders followed by gastrointestinal disorders (25). Nausea (19 Patients) being the most commonly reported ADR. Apart from those mentioned in Table 2, a few other associated ADRs were also reported which include weight loss, blurring of vision, unpleasant feeling and pulling sensation in the lower limbs, etc. Table 3 shows the Anatomical Therapeutic Chemical (ATC) classification of the nervous system or neuropsychiatric drugs implicated in the causation of ADRs [10].
Table 2 Showing the SOC involved as a manifestation of ADRs
| SOC | Frequency | Adverse drug reactions |
|---|---|---|
| Skin and appendages disorders | 9 | Rash (4), Alopecia (2), Acneiform eruptions (1), Itching (1), Erosions with bullae (1), change in the texture of hair (1) |
| Gastrointestinal disorders | 25 | Nausea (19), Vomiting (16), Hypersalivation (9), Abdominal discomfort (5), Gastritis (3), Abdominal pain (2), Retrosternal burning (2), Gum hyperplasia (1) |
| Neurological disorders | 56 | Parkinsonism (8), Giddiness (7), Hypokinesia (6), Involuntary movements (7), Akathisia (6), Tremors (6), Acute dystonia (4), Oculogyric crisis (4), Slurred speech (3), Tardive dyskinesia (3), Burning sensation (3), Restless leg syndrome (1), Rigidity (1), Ataxia (1), Nystagmus (1), Headache (1). |
| Psychiatric disorders | 9 | Hypersomnia (6), Anorexia (5), Restlessness (3), Insomnia (1), Confusion (1) |
| Reproductive disorders | 1 | Delayed ejaculation (1), Painful Gynecomastia (1) |
| Body as a whole-general disorders | 4 | Generalized edema (1), lower limb edema (1), Pedal edema (2) |
Table 3 ATC classification of drugs acting on the nervous system with representative examples of drugs that have been suspected to be responsible for the ADRs
| ATC class of drugs | Individual drugs with several patients in which it has caused ADRs |
|---|---|
| Antiepileptics | Divalproex sodium (12), Phenytoin sodium (5), Valproate sodium (3), Carbamazepine (2), Phenobarbitone (1), Topiramate (1) |
| Psycholeptics | A. Antipsychotics- Aripiprazole (14), Amisulpride (13), Lurasidone (10), Risperidone (5), Haloperidol (4), Chlorpromazine (2), Olanzapine (2), Quetiapine (2), Penfluridol (1), Flupenthixol (1) |
| B. Anxiolytics | |
| C. Sedative and hypnotics: Clonazepam (1) | |
| Psychoanaleptics | A-Antidepressants-Escitalopram (10), Vilazodone (5), Sertraline (3), Amitriptyline (1), Clomipramine (1), Fluvoxamine (1), Desvenlafaxine (1) |
| B-Antidementia drugs- Donepezil+Memantine (1) | |
| Psycholeptics+psychoanaleptics | Paroxetine+Clonazepam (6) |
| Antiepileptics+psychoanaleptics | Amitriptyline+Pregabalin (1) |
Among the drugs incriminated, antipsychotics were the commonest group of agents causing ADRs followed by antidepressants and antiepileptics. Aripiprazole and Amisulpride were the commonest antipsychotic drugs implicated in the causation of ADRs. Causality assessment revealed 33 (31.7%) patients had ADRs belonging to the category of "probable", whereas 71 (68.3%) had ADRs of the "possible" category, according to the WHO-UMC scale. 66 (63.5%) patients had mild ADRs, 36 (34.6%) had moderate and 2 (1.9%) had severe ADRs according to the criterion developed by Hartwig et al.
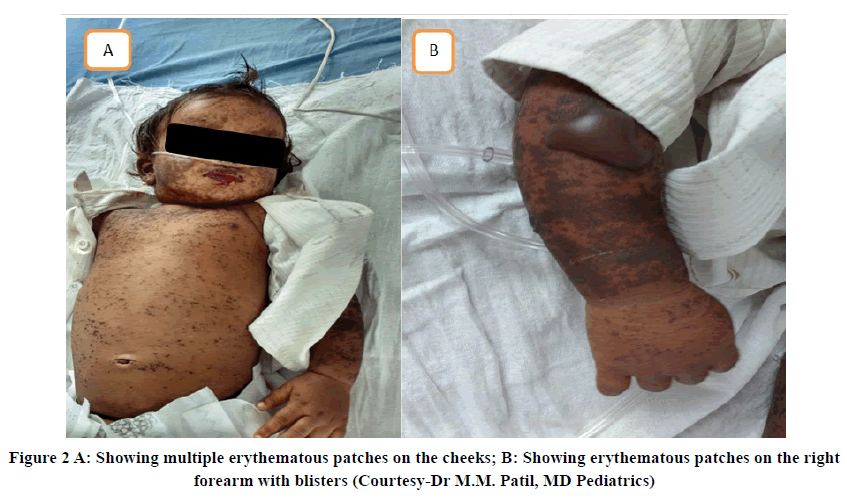
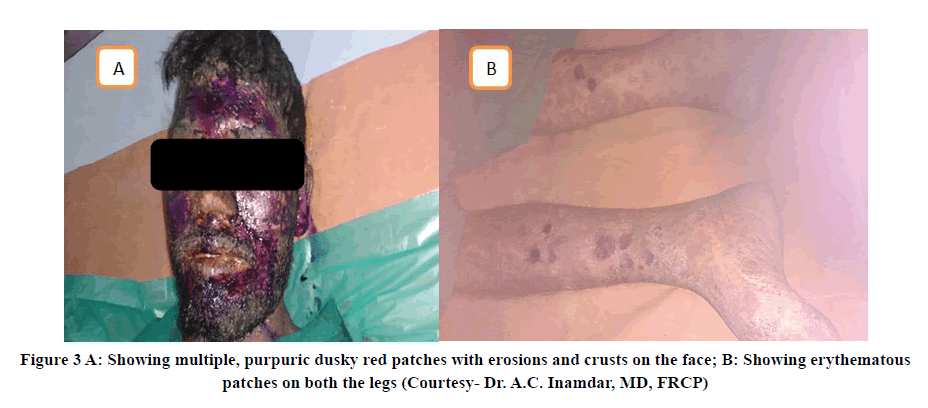
One severe ADR reported was Erythema multiforme secondary to the intake of Phenobarbitone (Gardenal sodium) 45 mg as shown in Figure 2. Another severe ADR was toxic epidermal necrolysis possibly induced by Phenytoin sodium as shown in Figure 3 [11].
Outcome and Preventability
56 (53.8%) patients had ADRs whose outcome was unknown. The remaining 38 (36.5%) recovered, 4 (3.8%) were recovering and 6 (5.8%) patients continued to have ADRs at the time of reporting. 48.07% of ADRs were preventable according to the Schumock-Thornton criteria.
Methods used to prevent ADR related morbidity:
- Discontinued or stopped the suspected medications
- Reduced the dose or changed the medication in certain scenarios
- Use of anticholinergics like Trihexyphenidyl and/or Benzodiazepines like Clonazepam
Discussion
The present study was conducted to find out the pattern of ADRs to neuropsychiatric drugs used in a teaching hospital of North Karnataka. The results of our study show that majority of the patients who had ADRs belong to the age group of 21-40 years. This can be explained because the majority of psychiatric illnesses will have their onset in the 2nd or 3rd decade of life. In comparison with the previous studies, our study didn't find any preponderance of the ADRs towards particular sex.
Most of them had Type A (Dose-related) reactions. Patients diagnosed with Schizophrenia and Bipolar affective disorder had most of the ADRs following treatment. Nausea being the most common ADR reported, as opposite of tremors reported by Sengupta, et al. and neurological disorders -was the commonest SOC involved, similar to the study by Prajapati, et al. [12,13]. Consistent with the previous Indian studies, a maximum number of ADRs were seen in patients who received antipsychotic drugs. Aripiprazole, Amisulpride, and Lurasidone were commonly prescribed and suspected antipsychotic drugs in our study, as opposed to the study by Sengupta, et al. Most of the ADRs were mild to moderate in severity, two severe cutaneous adverse drug reactions caused by Phenobarbitone and Phenytoin were also reported. The outcome of the ADRs in the majority of the patients was not known. 48.07% of ADRs were preventable according to the Schumock criteria. To treat the ADRs especially those induced by antipsychotics-anticholinergics like trihexyphenidyl and benzodiazepines like clonazepam were used [14].
Limitations
Rechallenge was not performed due to ethical reasons by the attending physician. Hence, it is difficult to label an ADR as "Certain".
Further research in this area is needed because of the approval of a large number of atypical antipsychotics and newer antiepileptics.
Conclusion
The present study adds to the existing information on the pattern of ADRs following administration of the drugs useful in the treatment of neuropsychiatric conditions. Although we have adopted passive surveillance method such as spontaneous reporting for ADR monitoring, which often underreporting is very common. Hence, it is difficult to find out or create a complete profile of adverse effects of recently approved drugs. We need to perform active surveillance of the drug-related injury (event), especially in the post-marketing phase for neuropsychiatric drugs. As it can generate few newer safety signals, which would have been missed or not detected during the clinical trial. Building a robust database for newer drugs will help the physician to choose safe and effective medicines for the treatment of neuropsychiatric illnesses.
Declarations
Conflicts of Interest
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Acknowledgments
I would like to thank NCC-PvPI, IPC, Ministry of Health, and Family Welfare for providing training and technical support to Shri B.M. Patil Medical College and the clinical staff, nurses of Shri B.M Patil Medical College Hospital for contributing to data collection.
References
- Edwards, I. Ralph, and Jeffrey K. Aronson. "Adverse drug reactions: Definitions, diagnosis, and management." The Lancet, Vol. 356, No. 9237, 2000, pp. 1255-59.
- Lazarou, Jason, Bruce H. Pomeranz, and Paul N. Corey. "Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies." JAMA, Vol. 279, No. 15, 1998, pp. 1200-05.
- Ganguli, H. C. "Epidemiological findings on prevalence of mental disorders in India." Indian Journal of Psychiatry, Vol. 42, No. 1, 2000, pp. 14-20.
- Lucca, Jisha Myalil, et al. "Identification and management of adverse effects of antipsychotics in a tertiary care teaching hospital." Journal of Research in Pharmacy Practice, Vol. 3, No. 2, 2014, pp. 46-50.
- Singh, Hemendra, Mariya Yacob, and Letty Sabu. "Adverse drug reactions monitoring of psychotropic drugs: A tertiary care centre study." Open Journal of Psychiatry & Allied Sciences, Vol. 8, No. 2, 2017, pp. 136-40.
- World Health Organization. "The Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment, 2018." 2020.
- Schumock, G. T., and J. P. Thornton. "Focusing on the preventability of adverse drug reactions." Hospital Pharmacy, Vol. 27, No. 6, 1992, pp. 538-38.
- Hartwig, Steven C., Jerry Siegel, and Philip J. Schneider. "Preventability and severity assessment in reporting adverse drug reactions." American Journal of Hospital Pharmacy, Vol. 49, No. 9, 1992, pp. 2229-32.
- Nahler, Gerhard. "WHO-Adverse Reaction Terminology (WHO-ART)." Dictionary of Pharmaceutical Medicine, 2009, pp. 192-93.
- WHO Collaborating Centre for Drug Statistics Methodology. "ATC/DDD Index 2021." https://www.whocc.no/atc_ddd_index/
- Bahrani, Eman, et al. "Cutaneous adverse effects of neurologic medications." CNS Drugs, Vol. 30, No. 3, 2016, pp. 245-67.
- Sengupta, Gairik, et al. "Adverse drug reaction monitoring in psychiatry out-patient department of an Indian teaching hospital." Indian Journal of Pharmacology, Vol. 43, No. 1, 2011, pp. 36-39.
- Prajapati, Hiren K., et al. "Adverse drug reaction monitoring in psychiatric outpatient department of a tertiary care hospital." Depression, Vol. 63, 2013, pp. 15-48.
- Tandon, Vishal R., et al. "Under-reporting of adverse drug reactions: A challenge for pharmacovigilance in India." Indian Journal of Pharmacology, Vol. 47, No. 1, 2015, pp. 65-71.