Research - International Journal of Medical Research & Health Sciences ( 2021) Volume 10, Issue 4
Phenotypic Characterization of Acinetobacter spp. Isolated from Ventilator-Associated Pneumonia in an Intensive Care Unit of Tertiary Care Hospital
Vijeta Sharma*, Rajni Sharma and Aruna VyasVijeta Sharma, Department of Microbiology, S.M.S Medical College and Attached Hospitals, Jaipur, Rajasthan, India, Email: 88vijeta@gmail.com
Received: 05-Apr-2021 Accepted Date: Apr 23, 2021 ; Published: 30-Apr-2021
Abstract
Acinetobacter spp. has been recognized as one of the important causes of hospital-acquired infection in Ventilator- Associated Pneumonia (VAP). The emergence of Multidrug Resistance (MDR) Acinetobacter spp. has shown resistance to most available antibiotics especially in the intensive care unit. A total of 100 Acinetobacter spp were collected from 272 endotracheal aspirates among the VAP patients. The Kirby Bauer Disk Diffusion Method was used to conduct an antibiotic susceptibility test on quantitative cultures. The Phenotypic Confirmatory Disk Diffusion Test (PCDDT) was used to classify ESBL producers. Combined Disk Test detected MBL producers. Carbapenemase activity was detected by Modified Hodge Test (MHT) and these strains were subjected to E-Test for further confirmation of carbapenemase activity. Acinetobacter spp were found highly resistant to most of the cephalosporin but 100% sensitive to Polymixin B and Tigecycline. Out of 100 isolates of Acinetobacter spp., 41 bacterial isolates were detected as ESBL producers and 44 isolates were AmpC Producers. 48 isolates were resistant to Imipenem. Out of 48 isolates, 40 were CDT positive and all 48 isolates were MHT positive. All MHT positive strains were subjected to E-Test. All isolates which were MHT positive were also E-Test positive. This study determined Metallo β-Lactamase produced by Acinetobacter spp. isolates. This can assist in the development of effective therapeutic strategies for VAP due to Acinetobacter spp.
Keywords
Ventilator-associated pneumonia, Multidrug resistance, Phenotypic confirmatory disk diffusion test, Combined disk test, Modified Hodge Test
Introduction
VAP is pneumonia that develops in people who have been on mechanical ventilation for over 48 hrs through an endotracheal or tracheotomy tube. A high mortality rate is associated with it [1].
Acinetobacter spp. is an occasional bacterial pathogen correlated mostly with hospitals acquired infection. The bacil- lus is a gram-negative, non-motile, non-lactose fermenter with a high prevalence of people living in immune-compro- mised stage with prolonged hospital stay [2]. Acinetobacter spp. are the commonest cause of late-onset VAP and have a higher than most bacteria mortality [3].
Acinetobacter spp. emerged as a main nosocomial and opportunistic disease because it is survival potential, omnipres- ent nature, and rapid development in antimicrobial resistance [4]. Therefore, the treatment of Acinetobacter spp. has become challenges due to acquiring resistance to various antibiotics through the inherent and acquired mechanism. Carbapenem was once the treatment of choice for VAP but, carbapenem can be hydrolyzed in various antibiotics including penicillin, cephalosporin, and carbapenems by the family of β-lactamases known as Metallo β-lactamases (MBL) [5-7]. This research was conducted with various phenotypic methods for MBL identification.
Material and Methods
This study was carried out in the Department of Microbiology, SMS Medical College and Attached Hospitals, Jaipur (Rajasthan) from Nov 2017 to Nov 2020. One hundred Acinetobacter spp. isolated from endotracheal aspirates of VAP patients which fulfilled the inclusion criteria were collected.
Inclusion Criteria
• Mechanical ventilation patients in ICU for over 48 hours
• Fever greater than 38.5°C
• Leukopenia (≤ 4,000 cells/mm3) or Leukocytosis (≥ 10,000 cells/mm3)
• Radiograph infiltrates
• All tracheal aspirate samples containing Acinetobacter isolates in significant >105 cfu/ml
The following method was used to detect the antimicrobial resistance mechanism among multidrug resistance Aci- netobacter spp
Phenotypic Confirmatory Disk Diffusion Test (PCDDT): The test isolate was swabbed on the MHA plate. A disc of ceftazidime (30 µg) in combination with clavulanic acid (30/10 µg) was placed at a distance of 20 mm (center to center) and incubated at 37°C. The result was interpretive as ESBL positive if the inhibition zone is ≥ 5 mm increase in inhibition halo compared with a disk without clavulanic acid. Klebsiella pneumoniae 700603 was used as a control strain for a positive ESBL production and Escherichia coli 25922 was used as a negative control for the ESBL pro- duction.
Inclusion Criteria
• Mechanical ventilation patients in ICU for over 48 hours
• Fever greater than 38.5°C
• Leukopenia (≤ 4,000 cells/mm3) or Leukocytosis (≥ 10,000 cells/mm3)
• Radiograph infiltrates
• All tracheal aspirate samples containing Acinetobacter isolates in significant >105 cfu/ml
Exclusion Criteria
• Patients within 48 hours with artificial ventilation
• Patients having pulmonary infiltrate before mechanical ventilation
• Exclusion in duplication of therapeutic isolates. Also omitted are all isolates that contain mixed organisms including Acinetobacter
Collection of Sample
Endotracheal aspirates collected using 22 inches Ramson's 12F mucus extractor suction catheter was introduced gently throughout the endotracheal strip for an approximate distance of 25 cm. Then the catheter was removed and a gentle aspiration was carried out without instilling saline [8]. Samples have been taken to the testing laboratory immediately for microbiological analysis. Gram's staining and quantitative cultures were subjected to samples.
Mechanically homogenize and serially diluted samples in the quantitative culture process by final dilutions of 10-2, 10-3, and 10-4 in 0.9 percent sterile saline solution. Blood Agar (BA) and MacConkey Agar (MA) samples were cul- tivated and overnight incubated at 37°C. Acinetobacter spp. identification was performed based on the laboratory procedure phenotypic criteria. Antimicrobial susceptibility test was performed on MHA by Kirby Bauer disk diffu- sion method for the following antimicrobial agents as per CLSI guidelines [9] Piperacillin-tazobactam (100/10 µg), ceftazidime (30 µg), cefotaxime (30 µg), cefepime (30 µg), imipenem (10 µg), gentamicin (10 µg), amikacin (30 µg), doxycycline (30 µg), polymyxin B and tigecycline [9]. ATCC 25922 and ATCC 27853 were used as the quality control strains like Escherichia coli and Pseudomonas aeruginosa.
The following method was used to detect the antimicrobial resistance mechanism among multidrug resistance Aci- netobacter spp
Detection of ESBL
Phenotypic Confirmatory Disk Diffusion Test (PCDDT): The test isolate was swabbed on the MHA plate. A disc of ceftazidime (30 µg) in combination with clavulanic acid (30/10 µg) was placed at a distance of 20 mm (center to center) and incubated at 37°C. The result was interpretive as ESBL positive if the inhibition zone is ≥ 5 mm increase in inhibition halo compared with a disk without clavulanic acid. Klebsiella pneumoniae 700603 was used as a control strain for a positive ESBL production and Escherichia coli 25922 was used as a negative control for the ESBL pro- duction.
Detection of AmpC Producers
• AmpC Production: By using the lawn culture process, the test organism was inoculated on MHA plates. Cefoxitin disc was applied to the media. Plates were incubated at 35°C-37°C for 24 hrs and zone size was measured with a ruler. AmpC beta-lactamase manufacturer was construed as the inhibition area size ≤ 18 mm across the cefoxitin disc.
•AmpC Disc Test: A lawn culture of Escherichia coli ATCC 25922 strain was made on Mueller-Hinton agar plate. Cefoxitin disc (30 μg) was placed on the inoculated MHA plate. AmpC disc was prepared by moistened the blank disc with 20 μl of sterile saline and inoculated with colonies of the test organism. The disc was placed beside the cefoxitin disc (almost touching) with the inoculated side facing downward. Then the plate was incubated at 35°C-37ºC for 24 hrs. There was if flattening or indentation of cefoxitin inhibition zone considered as AmpC producer [10].
Detection of MBL: MBL production would be screened by:
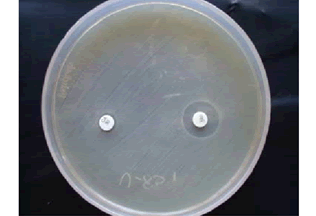
• Combined Disc Test (CDT): The test culture was made on the MHA as per CLSI guidelines [9]. Commercially available imipenem (10 μg) and imipenem/EDTA (10+750 μg) discs were placed on MHA at a distance of 20 mm each other. After an incubation period of 16 hrs-18 hrs at 37°C, the increase in inhibition zone ≥ 7 mm around the IMP/EDTA disc than IMP alone was considered as MBL positive [11].
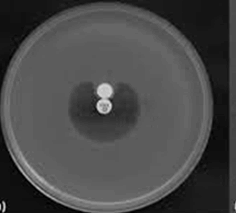
• Modified Hodge Test: This test was conducted to detect carbapenemase [12]. An overnight culture of E. coli ATCC 25922 adjusted to 0.5 McFarland standard was inoculated on MHA. An imipenem disc of 10 μg concentration was placed on the center of the plate and test culture was streaked from the edge of the disc to the periphery of the plate. After an incubation period of 16-18 hours at 37°C, the presence of cloverleaf zone or distortion of inhibition around the imipenem disc was screened for the presence of the carbapenemase.
• MBL E-Test: To detect the minimum inhibitory concentration ratio and to confirmative MBL production, all imipenem resistance isolates (Ezy MICTM Strip, Himedia) were E-tested. E-test MBL strains containing the seven imipenem (4 μg/ml-256 μg/ml) and the Imp-EDTA (1 μg/ml-64 μg/ml) double-sided dilution range. The MIC ratio >8 for the two reagent sides indicates MBL producers [13].
Results
A total of 272 endotracheal aspirates were received, out of which 100 Acinetobacter spp. were identified from these specimens. Acinetobacter spp. was the most common organism among the VAP patients. The disc diffusion suscep- tibility result for Acinetobacter spp. shows higher resistance pattern towards ceftazidime (98%), cefotaxime (98%), cefepime (95%), gentamicin (92%), amikacin (82%), ciprofloxacin (85%), and pipercilline+tazobactam (85%). Aci- netobacter spp. shows moderate resistance towards imipenem (51%) and it was 100% sensitive to polymixin B and tigecycline.
Out of 100, 41% isolates were ESBL producers by PCDDT, 44% were AmpC producers. Out of 48 imipenem resistant isolates, 40% of isolates were detected as MBL producers by CDT. 48% were MHT positive. All MHT positive strains were positive in E-Test (Table 1, and Figures 1-6).
| Organism | ESBL | AmpC | CDT for MBL | MHT for carbapenemase | E-Test |
| Acinetobacter spp. (100) | 41 (41%) | 44 (44%) | 40 (83.3%) | 48 (100%) | 48 (100%) |
Discussion
Acinetobacter spp. are the general c ause of ventilator-associated pneumonia. As this organism is still dry and moist and sometimes results in nosocomial infections, it persists for a long period [14,15]. The MDR organisms raise the probability of mortality in patients as they develop rapid resistance to the different groups of the antimicrobials includ- ing aminoglycosides, fluoroquinolones, and carbapenems [16].
In the present study the Acinetobacter spp. (36.76%) was the commonest causative agent associated with VAP. A similar finding was reported by Goel, et al. (36.82%), Khatun, et al. (32.5%) and Wajhat, et al. (36.11%) [17-19]. Acinetobacter spp. can resist disinfectant and survival longer even on dry surfaces. Therefore, patients on prolonged mechanical ventilation are more prone to VAP.
In our study, Acinetobacter spp. found higher among male patients (65%) than female (35%). Similar findings were reported by Rana, et al. (66.7% male and 33.3% Female), Daef, et al. (65.79% male and 34.21% female) and Verma, et al. (60.71% male and 39.28% female) [20-22].
VAP due to Acinetobacter spp. was more common in 41-60 years age group patients. Similar findings were reported by Patro, et al. (41-60 years), Patil, et al. (>40 years) and Golia, et al. (40-60 years) [23-25].
In the present study, 47% of patients had early VAP and 53% had late VAP. Similar findings were reported with Verma, et al. (43.66% early-onset and 56.33% late-onset), Joseph, et al. (41.7 early-onset and 58.3 late-onset), and Golia, et al. (44.23 early-onset and 55.77% late-onset) [22,25,26].
In our study low incidence of early onset of VAP was due to prior antimicrobial therapy before admission in ICU and late-onset of VAP contributed to higher infection of MDR pathogens.
In our study higher resistance were seen to cephalosporin (ceftazidime 98%, cefotaxime 98%, cefepime 95%), aminoglycoside (gentamicin 92% and amikacin 82%), fluoroquinolones (ciprofloxacin 85%) and β-lactamase inhibitor (pipercilline+tazobactam 85%). Our results were in concordance with the study of Goel, et al. (ceftazidime and cefepime 100%, gentamicin 100% and amikacin 99.9%, ciprofloxacin 98.4%, pipercilline+tazobactam 98.5%), Rana, et al. (100% resistant to ceftazidime, cefepime, gentamicin, amikacin ciprofloxacin and pipercilline+tazobactam), and Samal, et al. (ceftazidime 81% and cefepime 90%, gentamicin 81%) [26,27,28]. Imipenem resistance was found in 48% isolates of Acinetobacter spp., similar findings were observed in the study of Goel, et al. (44%), Golia, et al. (37.5%) and Srivastava, et al. (28.9%) [28,27,29]. The resistance to imipenem was higher in the study of Rana, et al. (91.2%) which may be due to carbapenems have been extensively used [20].
In our study higher sensitivity was seen to polymixin B and tigecycline (100% sensitivity), our findings are consistent with the study of Jethwani, et a1. (100% sensitive) and Goel, et al. (100% sensitive) [27,30].
The multi-drug resistance of the Acinetobacter spp. was caused by ESBL, AmpC, and MBL. In the present study, ESBL was found 41%, our result is in concordance with the study of Vijaykumar, et al. (41.5%), Goel, et al. (32.9%) and Rit, et al. (33.3%) [17,31,32]. A high incidence of ESBL production was reported by Neupane, et al. (57.14%) and Pragasam, et al. (71%) [33,34]. Variations in results were due to geographical differences among the different patients studied and different rates of antibiotics used in different hospitals [35]. AmpC producers were observed 44% which showing similarities with Rana, et al. (47%) and Goel, et al. (56.66%) [20,27]. Out of 48 imipenem resistance isolates, Metallo β-lactamase were detected in our study was 40 (83.3%) by CDT and 48% by MHT (100%) and E-strip (100%) method. Our result in concordance with the Shivaprasad, et al. (81.1% CDT, 100% MHT, 100% E-Test) and Niranjana, et al. (96.6% MHT and 96.6% E-Test) [36,37].
In a comparison of CDT, MHT, and E- Test, E-Test was found as the most sensitive test. Although in our study MHT and E-test both were effective. MHT was a simple method for screening MBL. Perhaps there is nevertheless wrong false positivity on isolates due to mild AmpC enzyme carbapenem hydrolysis [36]. High positivity in MHT was in concordance with Amudhan, et al. [38]. Mechanisms for chromosomally or plasmid-controlled and highly sensitive testing in detecting MBL can be detected by E-Test MBL strips.
ESBLs are of increasing clinical concern. MBL isolates produce the most β-lactam agents and can disperse and hydrolyze them. Accurate detection by routine laboratory examination of this resistance phenotype is essential to implement proper infection control practices and initiate adequate empirical therapy [36].
Conclusion
In conclusion, the present study showed MDR Acinetobacter spp. is the most common pathogen associated with VAP especially in late-onset infections. In our study higher sensitivity was seen to polymixin B and tigecycline and the multi-drug resistance of the Acinetobacter spp. was caused by ESBL, AmpC, and MBL β-lactamase. MHT and E-Test for MBL detection are sensitive according to our study. Hence, the simultaneous existence of different carbapenem- resistant enzymes should be seriously considered to make newer strategies of therapeutic, infection control measures, and continuous surveillance.
Declarations
Conflicts of Interest The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
References
- Wood, G. Christopher, et al. "Comparison of ampicillin-sulbactam and imipenem-cilastatin for the treatment of Acinetobacter ventilator-associated pneumonia." Clinical Infectious Diseases, Vol. 34, No. 11, 2002, pp. 1425-30.
- Turton, Jane F., et al. "Comparison of Acinetobacter baumannii isolates from the United Kingdom and the United States that were associated with repatriated casualties of the Iraq conflict." Journal of Clinical Microbiology, Vol. 44, No. 7, 2006, pp. 2630-34.
- Baraibar, Jorge, et al. "Risk factors for infection by Acinetobacter baumannii in intubated patients with nosocomial pneumonia." Chest, Vol. 112, No. 4, 1997, pp. 1050-54.
- Nerad, J. L., et al. "Miscellaneous gram-negative bacilli: Acinetobacter, Cardiobacterium, Actinobacillus, Chromobacterium, Capnocytophaga, and others." Infectious Diseases, Vol. 3, 1998, pp. 1871-87.
- Van Looveren, M., H. Goossens, and ARPAC Steering Group. "Antimicrobial resistance of Acinetobacter spp. in Europe." Clinical Microbiology and Infection, Vol. 10, No. 8, 2004, pp. 684-704.
- Navon-Venezia, Shiri, Ronen Ben-Ami, and Yehuda Carmeli. "Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting." Current Opinion in Infectious Diseases, Vol. 18, No. 4, 2005, pp. 306-13.
- Nordmann, P., and L. Poirel. "Emerging carbapenemases in Gram-negative aerobes." Clinical Microbiology and Infection, Vol. 8, No. 6, 2002, pp. 321-31.
- Dey, Arindam, and Indira Bairy. "Incidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: A nine months' prospective study." Annals of Thoracic Medicine, Vol. 2, No. 2, 2007, pp. 52-57.
- Wayne, P. A. "Clinical and laboratory standards institute: Performance standards for antimicrobial susceptibility testing: 27th informational supplement." CLSI document M100-S27, 2017.
- Black, Jennifer A., Ellen Smith Moland, and Kenneth S. Thomson. "AmpC disk test for detection of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking chromosomal AmpC β-lactamases." Journal of Clinical Microbiology, Vol. 43, No. 7, 2005, pp. 3110-13.
- Yong, Dongeun, et al. "Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp." Journal of Clinical Microbiology, Vol. 40, No. 10, 2002, pp. 3798-801.
- Lee, Kyungwon, et al. "Modified Hodge and EDTA-disk synergy tests to screen metallo-(beta)-lactamase-producing strains of Pseudomonas and Acinetobacter species." Clinical Microbiology and Infection, Vol. 7, No. 2, 2001, pp. 88-91.
- Farshadzadeh, Zahra, et al. "Wide distribution of carbapenem resistant Acinetobacter baumannii in burns patients in Iran." Frontiers in Microbiology, Vol. 6, 2015, p. 1146.
- Paterson, David L. "The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species." Clinical Infectious Diseases, Vol. 43, No. Supplement_2, 2006, pp. S43-S48.
- Lortholary, Olivier, et al. "Nosocomial acquisition of multiresistant Acinetobacter baumannii: Risk factors and prognosis." Clinical Infectious Diseases, Vol. 20, No. 4, 1995, pp. 790-96.
- Peleg, Anton Y., Harald Seifert, and David L. Paterson. "Acinetobacter baumannii: Emergence of a successful pathogen." Clinical Microbiology Reviews, Vol. 21, No. 3, 2008, pp. 538-82.
- Goel, Nidhi, Parul Punia, and Uma Chaudhary. "Prevalence of ESBL, MBL and Amp C producing XDR Acinetobacter isolates from lower respiratory tract specimen." International Journal of Contemporary Medical Research, Vol. 4, No. 10, 2017, pp. 2091-95.
- Khatun, Nazma, et al. "Molecular characterization and resistance profile of nosocomial Acinetobacter baumannii in intensive care unit of tertiary care hospital in Bangladesh." Bangladesh Medical Research Council Bulletin, Vol. 41, No. 2, 2015, pp. 101-07.
- Ahmed, Wajahat. "Microorganisms related with Ventilator Associated Pneumonia (VAP) and their antibiotic sensitivity pattern." Journal of Rawalpindi Medical College, Vol. 18, No. 1, 2014, pp. 45-48.
- Rana, G., S. Sharma, and C. Hans. "Ventilator-associated pneumonia in the ICU: Microbiological profile." Journal of Bacteriology and Mycology, Vol. 4, No. 5, 2017, pp. 165-68.
- Daef, Enas A., et al. "Role of bacteriological investigation of endotracheal aspirate in diagnosis of ventilator-associated pneumonia." African Journal of Microbiology Research, Vol. 10, No. 28, 2016, pp. 1072-79.
- Verma, Lalita, et al. "Microbiological profile of ventilator associated pneumonia in a tertiary care intensive care unit." Indian Journal of Applied Research, Vol. 6, No. 7, 2016, pp. 43-46
- Patro, Somi, et al. "Bacteriological profile of ventilator-associated pneumonia in a tertiary care hospital." Indian Journal of Pathology and Microbiology, Vol. 61, No. 3, 2018, pp. 375-79.
- Patil, Harsha V., and Virendra C. Patil. "Incidence, bacteriology, and clinical outcome of ventilator-associated pneumonia at tertiary care hospital." Journal of Natural Science, Biology, and Medicine, Vol. 8, No. 1, 2017, pp. 46-55.
- Golia, Saroj, K. T. Sangeetha, and C. L. Vasudha. "Microbial profile of early and late onset ventilator associated pneumonia in the intensive care unit of a tertiary care hospital in Bangalore, India." Journal of Clinical and Diagnostic Research: JCDR, Vol. 7, No. 11, 2013, pp. 2462-66.
- Joseph, Noyal Mariya, et al. "Ventilator-associated pneumonia in a tertiary care hospital in India: Role of multi-drug resistant pathogens." The Journal of Infection in Developing Countries, Vol. 4, No. 04, 2010, pp. 218-25.
- Goel, Varun, Sumati A. Hogade, and S. G. Karadesai. "Prevalence of extended-spectrum beta-lactamases, AmpC beta-lactamase, and metallo-beta-lactamase producing Pseudomonas aeruginosa and Acinetobacter baumannii in an intensive care unit in a tertiary care hospital." Journal of the Scientific Society, Vol. 40, No. 1, 2013, pp. 28-31.
- Samal, Neha, Sanghamitra Padhi, and Bimoch P. Paty. "Bacteriological profile and antimicrobial sensitivity pattern of endotracheal tube aspirates of patients admitted in ICU." Journal of Dr. NTR University of Health Sciences, Vol. 9, No. 3, 2020, pp. 151-57.
- Shrivastava, Gunjan, et al. "Sensitivity profile of multidrug resistant Acinetobacter Spp. isolated at ICUs of tertiary care hospital." International Journal of Health System and Disaster Management, Vol. 1, No. 4, 2013, pp. 200-03.
- Jethwani, Urmi, Pranav Trivedi, and Neelam Shah. "Antibiotic sensitivity pattern of Gram negative bacilli isolated from the lower respiratory tract of ventilated patients in the intensive care unit." Indian Medical Gazette, 2014.
- Vijayakumar, Saranya, et al. "Molecular characterization of invasive carbapenem-resistant Acinetobacter baumannii from a tertiary care hospital in South India." Infectious Diseases and Therapy, Vol. 5, No. 3, 2016, pp. 379-87.
- Rit, Kalidas, et al. "Ventilator associated pneumonia in a tertiary care hospital in India: Incidence, etiology, risk factors, role of multidrug resistant pathogens." International Journal of Medicine and Public Health, Vol. 4, No. 1, 2014, pp. 51-56.
- Neupane, Mary, et al. "Beta-lactamases production in multi-drug resistant Acinetobacter species isolated from different clinical specimens." Tribhuvan University Journal of Microbiology, Vol. 6, No. 1, 2019, pp. 44-50.
- Pragasam, A. K., et al. "Molecular characterisation of antimicrobial resistance in Pseudomonas aeruginosa and Acinetobacter baumannii during 2014 and 2015 collected across India." Indian Journal of Medical Microbiology, Vol. 34, No. 4, 2017, pp. 433-41.
- Abd El-Baky, Rehab M., et al. "Antimicrobial resistance pattern and molecular epidemiology of ESBL and MBL producing Acinetobacter baumannii isolated from hospitals in Minia, Egypt." Alexandria Journal of Medicine, Vol. 56, No. 1, 2020, pp. 4-13.
- Shivaprasad, Aparna, Beena Antony, and Poornima Shenoy. "Comparative evaluation of four phenotypic tests for detection of metallo-β-lactamase and carbapenemase production in Acinetobacter baumannii." Journal of Clinical and Diagnostic Research: JCDR, Vol. 8, No. 5, 2014, p. DC05.
- Niranjan, D., et al. "Multiple carbapenem hydrolyzing genes in clinical isolates of Acinetobacter baumannii." Indian Journal of Medical Microbiology, Vol. 31, No. 3, 2013, pp. 237-41.
- Amudhan, M. Shanthi, et al. "blaIMP and blaVIM mediated carbapenem resistance in Pseudomonas and Acinetobacter species in India." The Journal of Infection in Developing Countries, Vol. 6, No. 11, 2012, pp. 757-62.