Research - International Journal of Medical Research & Health Sciences ( 2023) Volume 12, Issue 3
Role of 0.01% Atropine in Progressive Myopia in Children
Jai Shree Singh, Sangam* and Harlal SinghSangam, Government medical college, Rajasthan, India, Email: sangam127306@gmail.com, sangamgahlawat24477@gmail.com
Received: 27-Feb-2023, Manuscript No. ijmrhs-23-90304; Editor assigned: 09-Mar-2023, Pre QC No. ijmrhs-23-90304(PQ); Reviewed: 11-Mar-2023, QC No. ijmrhs-23-90304(Q); Revised: 13-Mar-2023, Manuscript No. ijmrhs-23-90304 (R); Published: 28-Mar-2023
Abstract
Objective: Role of 0.01% atropine in progressive myopia in children. Material and methods: After getting approval from the ethical committee of the Government medical college kota, we conducted a prospective study of 50 children from march 2021 to march 2022 for progressive myopia (>0.5 D/year) out of which 25 children got treatment in form of topical atropine 0.01%. The effectiveness of the drug was evaluated by calculating SE (Spherical Equivalent) at every visit. Mean change in SE was calculated before treatment and after treatment and a comparison of both the mean values was done for the efficacy of the drug. Results: Out of 25 treatment groups, 14 were males and 11 were females. There was 13 male and 12 female in the control group. The mean age was 9.7 years ± 2.3 years (range 5 years-14 years) and 12.1 years ± 2.9 years (6 years-16 years) in the atropine and control groups respectively. At baseline mean SE was found to be -2.9 ± 0.149 and -2.63 ± 0.268 whereas Best Corrected Visual Acuity (BCVA) was 0.438 ± 0.067 and 0.65 ± 0.14 in the atropine and control group respectively. The rate of myopia progression in study participants. The mean progression rate was found to be lower in the atropine group when compared before and after treatment (-0.97 ± 0.055 versus -0.23 ± 0.018). It was found to be 0.23 D/year which is supported by various previous studies like the atom 2 study in which myopic rate progression was 0.42 D after 12 months of atropine use. Conclusion: It can be concluded that 0.01% atropine eyedrops used once daily before bed can slow the progression of myopia with very good tolerance and few side effects, making it a recommended treatment to be included in our therapeutic routine.
Keywords
Myopia, Best Corrected Visual Acuity (BCVA), Spherical Equivalent (SE)
Introduction
When the picture of a distant object is formed anterior to the retinal plane, which most frequently happens as a result of an increased axial length, myopia (near-sightedness) develops. This results in blurred distant vision and, unlike hyperopia, requires refractive correction at all ages and severity for clear focus [1]. Myopia is the most common ocular disorder, predominantly in East Asia. It is estimated that around 2.5 billion people have developed myopia by 2020, and approximately half of the world's population will become myopic, with 10% of them highly myopic by 2050 [2]. In addition to the decreased visual function from optical defocus, myopia is associated with an increased lifelong risk of irreversible blinding conditions such as myopic macular degeneration, retinal detachment, and glaucoma [3]. The cause and underlying mechanism of myopia progression remain unclear; therefore, its increasing prevalence is not well understood. Several theories have been proposed to explain the recent increase and its earlier onset in children, including a decrease in outdoor activity, an increase in time spent doing near work, and an increase in urbanization. Despite these theories and studies showing that increasing outdoor activity and decreasing near work may help to retard myopic progression [4]. Thus, myopia is a major public health concern, posing a heavy health and economic burden to society. Recently, outdoor activities and decreasing the duration of near work have been reported to be effective in delaying myopia onset. However, among the various interventions evaluated, atropine is one of the most consistently effective interventions in slowing down myopia progression.
Atropine eye drops were first proposed as a treatment for myopia in the 1920s. Since then, there have been numerous studies on this subject [5]. Atropine eye drops, a nonselective muscarinic antagonist, have been used for myopia control for some years. Numerous studies 13Y22 have demonstrated that atropine is effective in slowing myopia progression in children, although its side effects such as photophobia, blurred near vision, and systematic adverse effects are still sources of concern [6]. Initially, it was proposed that accommodation was a causative factor in myopia progression therefore cycloplegia following the use of atropine may cause retardation of myopia progression. But current theories state that atropine causes pupillary dilation which may result in increased UA exposure which in turn may limit axial elongation. the local retinal effect that may retard myopic progression or a potential biochemical change brought about by binding muscarinic receptors. A higher concentration of atropine (commonly 1%) was initially investigated and found to be substantially effective in slowing axial elongation between 70% and up to 94% in well-conducted trials [7].
But a higher dose (1%) has a rebound phenomenon with more side effects after cessation the authors suggested that 0.01% atropine is better in treatment-to-side affect balance.
Materials and Method
After getting approval from the ethical committee of the Government medical college kota, we conducted a prospective study of 50 children from march 2021 to march 2022 for progressive myopia (>0.5 D/year) out of which 25 children got treatment in form of topical atropine 0.01% [3].
Inclusion criteria for the study comprise of patients aged between 6 to 15 years with a myopic progression of 0.5 D in the past year (documented) with best corrected visual acuity ≥ 0.2 in each eye, treatment is done with 0.01% atropine eyedrop once at bedtime. Children with a history of use of any concomitant ocular medication during the treatment period, use of contact lenses within 3 days of examination, children with a disorder like glaucoma, strabismus, keratopathy, systemic disorders such as respiratory and cardiac illness, cataract, intraocular pressure >21 mm Hg, low birth weight, history of hypersensitivity to atropine, history of ocular surgery were excluded from the study [2].
Control for the study was chosen who met all the inclusion and exclusion criteria except they are not prescribed atropine and with myopic progression >0.5 D/year. They were also followed for 1 year along with cases
The effectiveness of the drug was evaluated by calculating SE (spherical equivalent) at every visit. Mean change in SE was calculated before treatment and after treatment and a comparison of both the mean values was done for the efficacy of the drug
Study Design
Best corrected visual acuity was noted in the decimal and a complete ophthalmic examination of all study participants was done at the initial presentation and after 6 months and 12 months of starting treatment.
Under cycloplegic condition refractive error of all patients was evaluated at baseline and after 12 months of treatment in treatment and control groups both using an autorefractor. Using refractive power spherical equivalent was determined at baseline, one year before starting the treatment and 12 months after the use of the drug. The spherical equivalent was calculated as sphere plus half cylinder. Subtracting the SE at baseline from SE at 1 year before treatment (rate of progression before treatment) and SE after 12 months of treatment from SE at baseline (rate of progression after treatment). Similarly, the rate of progression was calculated in the control group.
Treatment patients were considered as responders or nonresponders based on SE progression (responders if SE progression was, 0.5 D and nonresponders if progression was found to be >0.5 D).
Safety
We as ophthalmologists may also be concerned about the side effects of atropine. So all participants were informed about side effects and were asked about them at every visit. Among all treatment groups, the most common side effect was found to be photophobia (10%). Rest were having no major side effects that forced them to discontinue the treatment and overall the whole study was uneventful in terms of systemic side effects and atropine 0.01% was well tolerated.
Results
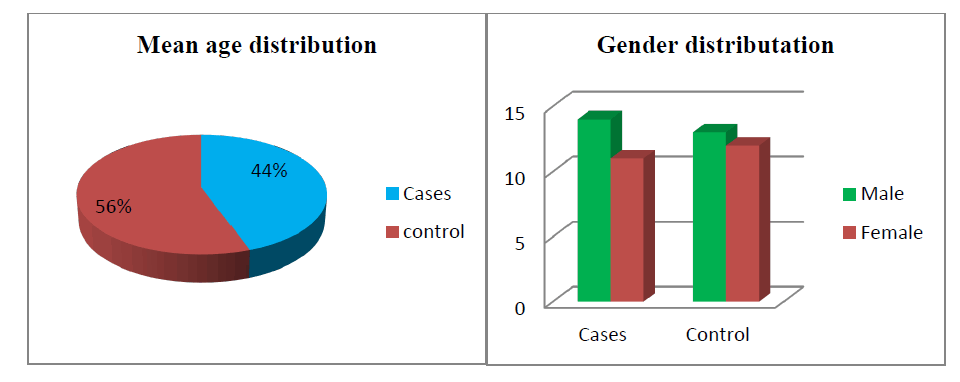
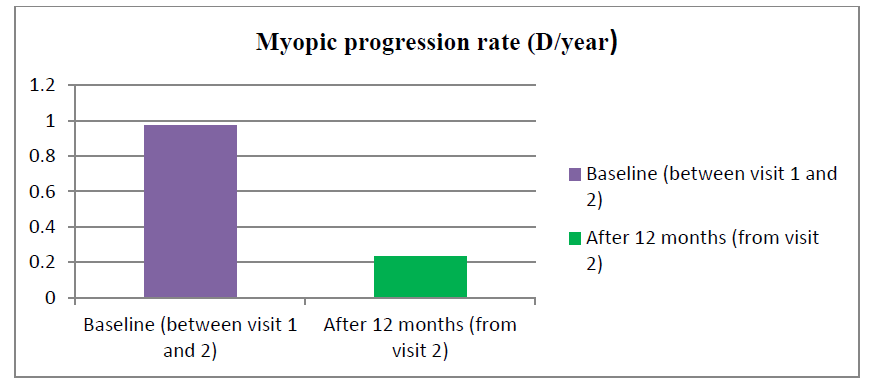
Out of 25 treatment groups, 14 were males and 11 were females. There was 13 male and 12 female in the control group. The mean age was 9.7 years ± 2.3 years (range 5 years-14 years) and 12.1 years ± 2.9 years (6 years-16 years) in the atropine and control groups respectively. At baseline mean SE was found to be -2.9 ± 0.149 and -2.63 ± 0.268 whereas Best Corrected Visual Acuity (BCVA) was 0.438 ± 0.067 and 0.65 ± 0.14 in the atropine and control group respectively (Table 1). Table 2 shows the rate of myopia progression in study participants. The mean progression rate was found to be lower in the atropine group when compared before and after treatment (-0.97 ± 0.055 versus -0.23 ± 0.018). It was found to be 0.23 D/year which is supported by various previous studies like the atom 2 study in which myopic rate progression was 0.42 D after 12 months of atropine use in Figure 1(a,b) and Figure 2.
| S.No. | Characteristic | Atropine 0.01 group | Control group | p-value | |
|---|---|---|---|---|---|
| 1 | Patients (n) | 25 | 25 | ||
| 2 | Gender | Male | 14 | 13 | |
| female | 11 | 12 | |||
| 3 | Mean Age (Mean ± 2SD) | 9.7 ± 2.3 (5-14 years) | 12.1 ± 2.9 (6-16) | 0.0022 | |
| 4 | Visit 1 | Spherical equivalence (SE) (Mean ± 2SD) | -2.91 ± 0.149 | -2.63 ± 0.268 | <0.0001 |
| BCVA (Mean ± 2SD) | 0.438 ± 0.067 | 0.65 ± 0.14 | <0.0001 | ||
| S. No. | Myopic progression rate (D/year) | Atropine 0.01 group | Control group | p-value |
|---|---|---|---|---|
| 1 | Baseline between visit 1 and visit 2 (Mean ± 2SD) | -0.97 ± 0.055 | -0.63 ± 0.14 | <0.0001 |
| 2 | After 12 months (Mean ± 2SD) | -0.23 ± 0.018 | -0.80 ± 0.36 | <0.0001 |
Discussion
We conducted a prospective study after getting clearance from the ethical committee of our institution. Our study enrolled a total of 50 participants whose myopic progression was >0.5 D /year and best corrected visual acuity was >0.2 decibels. 0ut of 50, 25 participants were prescribed topical atropine 0.01 % in both eyes to be instilled at bedtime after the complete ophthalmic examination. Rest 25 controls were the same in all aspects of the treatment group except they were not prescribed atropine. Out of 25 treatment groups, 14 were males and 11 were females. There was 13 male and 12 female in the control group. The mean age was 9.7 years ± 2.3 years (range 5 years-14 years) and 12.1 years ± 2.9 years (6 years-16 years) in the atropine and control groups respectively
Conclusion
It can be concluded that 0.01% atropine eyedrops used once daily before bed can slow the progression of myopia with very good tolerance and few side effects, making it a recommended treatment to be included in our therapeutic routine. However, it is essential to get the parents on board with using the treatment and to change habits to increase outdoor activities exposed to sunlight.
Declarations
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Williams, Katie M., et al. "Increasing prevalence of myopia in Europe and the impact of education." Ophthalmology, Vol. 122, No. 7, 2015, pp. 1489-97.
Google Scholar Crossref - Li, Fen Fen, and Jason C. Yam. "Low-concentration atropine eye drops for myopia progression." Asia-Pacific Journal of Ophthalmology, Vol. 8, No. 5, 2019, p. 360.
Google Scholar Crossref - Clark, Tiana Y., and Robert A. Clark. "Atropine 0.01% eyedrops significantly reduce the progression of childhood myopia." Journal of Ocular Pharmacology and Therapeutics, Vol. 31, No. 9, 2015, pp. 541-45.
Google Scholar Crossref - Pineles, Stacy L., et al. "Atropine for the prevention of myopia progression in children: a report by the American Academy of Ophthalmology." Ophthalmology, Vol. 124, No. 12, 2017, pp. 1857-66.
Google Scholar Crossref - Chia, Audrey, et al. "Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2)." Ophthalmology, Vol. 119, No. 2, 2012, pp. 347-54.
Google Scholar Crossref - Li, Shi-Ming, et al. "Atropine slows myopia progression more in Asian than white children by meta-analysis." Optometry and Vision Science, Vol. 91, No. 3, 2014, pp. 342-50.
Google Scholar Crossref - Chia, Audrey, Qing-Shu Lu, and Donald Tan. "Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops." Ophthalmology, Vol. 123, No. 2, 2016, pp. 391-99.
Google Scholar Crossref