Research - International Journal of Medical Research & Health Sciences ( 2022) Volume 11, Issue 6
Sgot and Sgpt Variations with their Pearsonâs Coefficient Correlation in Sickle Cell Disease
Ivvala Anand Shaker* and Jagdish Powar2Department of Biochemistry, Govt. Medical College and Hospital, Rajasthan, India
3Department of Biochemistry, Parul Institute of Medical Sciences & Research, Parul University, Vadodara, Gujarat, India
4Department of PSM, SMBT Institute of Medical Sciences and Research Centre, Maharashtra, India
Ivvala Anand Shaker, Department of Biochemistry, Parul Institute of Medical Sciences & Research, Parul University, Vadodara, Gujarat, India, Email: ianandshaker@rediffmail.com
Received: 18-Feb-2022, Manuscript No. ijmrhs-22-54885; Editor assigned: 21-Feb-2022, Pre QC No. ijmrhs-22-54885 (PQ); Reviewed: 22-Feb-2022, QC No. ijmrhs-22-54885 (Q); Revised: 12-May-2022, Manuscript No. ijmrhs-22-54885 (R); Published: 30-Jun-2022
Abstract
Background: Sickle cell disease is an inherited disorder caused by the single point mutation due to the replacement of valine for glutamic acid at the sixth codon of β-chain of globin’s (β6Glu-Val). The sickle cell disease condition with hepatic dysfunction shows abnormal variations in liver function tests including serum aminotransferases enzymes like Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) levels were changed to discuss their significance in sickle cell disease. Objectives: Study of SGOT and SGPT level variation in sickle cell disease. Study of Pearson’s correlation coefficient of Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) in sickle cell disease. Methods: This study was conducted in the Department of Biochemistry, People’s College of Medical Sciences and Research Centre (PCMS & RC), and Centre for Scientific Research and Development Department (CSRD), People’s University, Bhopal. The study protocol and ethical clearance had been approved by Institutional Ethical Committee (Reference number PCMS/OD/2016/2551). The sample size was estimated by the expert statistician, which was 111 for the case sample and controls. These were employed in the research study after the application of the inclusion and exclusion criteria. The liver function enzymes Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum glutamic pyruvic transaminase (SGPT) were estimated by Reitman & Frankel’s method and were diagnosed by using the Trans Asia diagnostic kits manufactured by Transasia Bio-Medicals Ltd., B-11, and OpenID Connect (OIDC). Ringanwada, Daman-396210 (India) in collaboration with ERBA diagnostics Mannheim HmbH Mallaustr. 69-73, D-68291, Mannheim/Germany. Results: In the present study, the mean standard deviation of cases vs controls showed a significant difference and it was calculated by using the SPSS latest software version-24. The Mean ± SD cases vs controls of Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum glutamic pyruvic transaminase (SGPT) were found in 69.98 ± 69.31, 25.17 ± 5.25 and 65.28 ± 60.07, 22.72 ± 5.47 respectively. The calculated P?0.000 was found to be statistically highly significant. The Pearson’s Correlation showed a positive correlation between Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) enzymes. Conclusions: The transaminases SGOT-SGPT enzymes were significantly increased in sickle cell disease so it could be another clinical biomarker for the diagnosis of sickle cell disease.
Keywords
Serum Glutamic-Oxaloacetic Transaminase (SGOT), Serum Glutamic Pyruvic Transaminase (SGPT), Pearson’s Correlation, Sickle Cell Disease
Introduction
Sickle cell disease is an inherited disorder caused by the single point mutation due to the replacement of glutamic acid with valine at the sixth codon of the β-chain of globins. (β6Glu-Val) which is an alternation of a single nucleotide base from thymine to adenine (GTG -GAG) the molecular change is responsible for the alternation in the properties of the hemoglobin tetramer, with a tendency to polymerize in the deoxygenated state altering normal, flexible, biconcave shaped Red Blood Cells (RBCs) into Stiff, Rigid, Sickle Cell RBCs. These changes are related to fundamental Pathophysiology, Vaso-Occlusion, Tissue Ischemia, sickle-shaped Erythrocytes, Leucocytes, Platelets, and endothelial cells [1,2].
Hepatic dysfunction is usually a known problem of sickle cell disease due to multiple factors such as intrahepatic sinusoidal sickling and transfusion-related hepatic infections [3].
The sickle cell disease conditions with hepatic dysfunction show abnormal variations in liver function tests including serum aminotransferases, bilirubin, and alkaline phosphatase [4]. Our study focuses on the clinical investigation of serum aminotransferase enzymes.
Methods
Place and Ethical Clearance of the Study
This study was conducted in the Department of Biochemistry, People’s College of Medical Sciences and Research Centre (PCMS and RC), and Centre for Scientific Research and Development Department (CSRD), People’s University, Bhopal. The study protocol and ethical clearance had been approved by the Research Advisory Committee (Ref. number PCMS/OD/2016/2566) and Institutional Ethical Committee (Ref. No PCMS/OD/2016/2551).
Sample Size Estimation
The sample size estimation had been carried out and confirmed by the expert statistician. The estimation was based on the prevalence of sickle cell disease in Madhya Pradesh, it was found in between the range of 15% to 30% [5].
The sample size estimation was carried out by using the formula N =4 PQ /L2
The estimated sample size was found to be 110.68. Hence 111 SCD sample cases or more samples are necessary to meet the desired statistical constraints and 111 healthy subjects were enrolled with their consent forms for the controls group in the study.
STUDY DESIGN
Type of Study
This is a Hospital-based, Case-control, prospective study.
Study Duration
The study sample collection had been started in the year 2019 and finished in December 2020.
Source of SCD Subjects
The blood samples for the study were collected from People’s Hospital and Civil Hospital Bairagarh, Bhopal, Madhya Pradesh.
Study Criteria
Inclusion criteria for sickle cell disease subjects:
• The subject has a diagnosis of sickle cell disease with other sickle cell-related complications alongside acute sickle pain, including not limited to acute chest syndrome, renal dysfunction, liver dysfunction stroke, vasoocclusive event, and priapism have been included in the study.
• Patients, more than 5 years of age and less than 70 years of age were included in the present study.
All the selected and enrolled patients had been confirmed with their specific characteristics with a single blood transfusion 3-4 months before blood was drawn. The patients with hospitalization were included in the study and might be or might not be under continuous Antibiotics therapy, and Hydroxyurea (HU) treatment. The patients under hydroxyurea treatment are advised to stop hydroxyurea for 15-20 days, and after that, the blood is to be collected for the research study.
Exclusion criteria for sickle cell disease subjects:
• Sickle cell subjects less than 5 years and more than 70 years were excluded from the study.
• Individuals with other hemoglobinopathy were also excluded from the study.
• SCD patients under chemotherapy treatment were excluded from the study.
• For the safety precautions in handling the blood samples, patients with HIV, Hepatitis B, and Hepatitis C will be excluded from the study. HIV, Hepatitis B, and Hepatitis C testing will not be done under this study.
Source of normal healthy subjects: The control group consists of 111 healthy normal individuals, healthy persons comprised of departmental staff, medical students, and relatives who were healthy and accompanied their OPD or IPD, and their health condition was being detected.
Inclusion criteria
• Healthy subjects more than 5 years of age and less than 70 years of age.
• Normal healthy individuals were recruited as a control group.
Exclusion criteria for control subjects
• Participants do not have a diagnosis of sickle cell disease or not any type of hemoglobinopathy.
• Healthy subjects less than 5 years of age and more than 70 years of age were excluded.
Informed Consent
The informed consent forms were obtained from the SCD patients and also from the healthy control group. All procedures followed were in accordance and approved by the Institutional Ethics Committee of the People’s College of Medical Sciences & Research Center, Bhopal, and also with the Helsinki Declaration of 1975 and its revisions.
Blood Sample Collection
The 5 ml overnight fasting venous blood had been collected from patients and controls under aseptic conditions. Then 5 ml of blood was taken into a plain vacutainer. Then the sample was centrifuged at 3000 rpm for 10 minutes for separation of the serum sample and then the separated sample was immediately stored in a deep freezer at 20ºC until it was used for further analysis.
Confirmation Tests for SCD
The following screening tests had been carried out with SCD patients for the confirmation of sickle cell RBCs.
• Sickling test with 2% meta-bisulfite: It is the principle of the sickling test, was based on Microscopically observation of sickling of red blood cells when exposed to low oxygen tension.
• Solubility test with 0.02% sodium dithionate: It is the principle of solubility method, based on turbidity created when Hb S is mixed with sodium dithionate.
• Peripheral blood film method: Thin blood films, stained with Giemsa stain was examined by light microscopy (100x).
• Hb electrophoresis cellulose acetate membrane Hb electrophoresis method had been used to determine the presence of Hb-S in the sample.
The liver function enzymes SGOT and SGPT were estimated by Reitman & Frankel’s method and were diagnosed by using the Trans Asia diagnostic kits. Manufactured by Transasia Bio-Medicals Ltd., B-11, and Oidc. Ringanwada, Daman-396210 (INDIA) in collaboration with ERBA diagnostics Mannheim HmbH Mallaustr. 69-73, D-68291, Mannheim/Germany [6].
Statistical Analysis
The statistical calculation was calculated by using the SPSS latest software version-24. We observed that Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) were found statistically highly significant in Sickle cell disease cases as compared to the controls. Pearson’s Coefficient Correlation shows us a positive Correlation with each other PË?0.000 was considered to be statistically highly significant.
Results
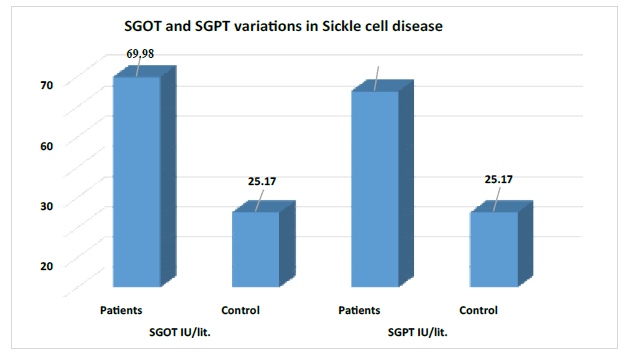
There was a highly significant difference observed between patients and controls of the above Serum GlutamicOxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) variables. Since the p<0.000 (Table 1).
| Variables | Group | N | Mean | SD | ‘T’value | ‘p’value | Significance level |
|---|---|---|---|---|---|---|---|
| SGOT IU/lit. | Patients | 111 | 69.98 | 69.31 | 6.79 | ˂0.000 | Highly |
| Control | 111 | 25.17 | 5.25 | significant | |||
| SGPT IU/lit. | Patients | 111 | 65.28 | 60.07 | 7.43 | ˂0.000 | Highly |
| Control | 111 | 25.17 | 5.25 | significant | |||
| (p˂0.0001= highly significant and p˂0.005 significant) | |||||||
Discussion
The activity of Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) has been widely used in liver function tests to assess liver conditions; both the enzymes are very sensitive and reliable for the assessment of liver function tests. These enzymes were increased in SCD due to the organ damage property of the sickling mechanism that causes liver dysfunction [7].
In our findings also, the liver enzymes Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) were found to increase significantly high in SCD cases but in the controls were found within the normal levels.
An increase in Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) is due to the rapid sickling of RBCs in sickle cell disease; the transamination was not completed so the enzymes were not utilized for the conversion of keto acids that leads to increases the transaminases SGOT and SGPT [8].
Our outcomes are correlated with Richard, et al. who stated that the statistically significant alterations in the levels of liver enzymes and other parameters were also seen in all the patients with SCD as compared to controls (p<0.0001), where the levels of LFT parameters significantly (p<0.0001) elevated in cases as compared to controls. But in steadystate sickle cell disease, Richard S, et al. studied the same variables in which he reported that there were no significant alterations in the levels of liver function enzymes in steady-state, which could be due to the steady-state of the patients where sickling and hemolysis are occurred [8].
Mahera, et al. and Gardner K, et al. concluded in their studies that, there was a significant increase in LFT parameters which were similar to both the results [9,10]. The similarities were observed in their studies and Gardner K, et al. also studied a milder increase of LFT was also observed in SCD cases [10].
Figure 1 designating that there was a severely increased level of Serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) found in the sickle cell disease patients as compared to the control.
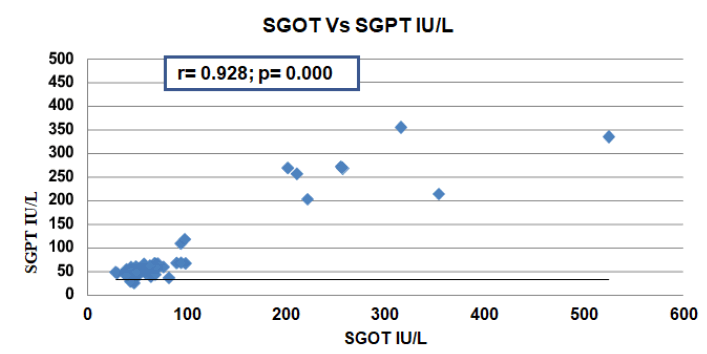
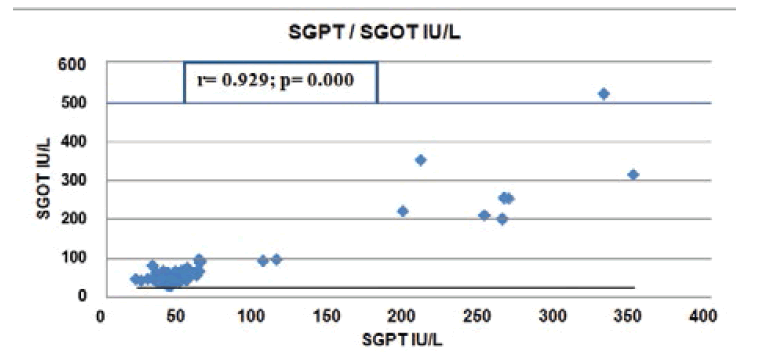
We have also studied by using Pearson’s Correlation Coefficient which showed a very significant positive correlation supported by the different articles discussed here below (Figure 2).
Akuyam, et al. studied in 2007 and they found that there were statistically highly significant increased levels of Total bilirubin, Sr. AST, Sr. ALT, and ALP in sickle cell disease Gardner K et al and Brody et al stated through the observations of his study that, the previous elevations of the Sr. AST (94.4%), ALT (2.8%), were found more in SCD patients Norris, et al. reported that the acute sickling process selectively affects the liver in 10% of the patients, causing a liver crisis with abdominal pain, nausea, fever, jaundice, and transaminases ( SGOT and SGPT) were found elevated in SCD [10-12].
Similarly, the scientists Johnson, Schubert, Sheehy, and Stephan stated that the liver enzyme transaminases were significantly increased in sickle cell hepatopathy, which was due to the rapid sickling and hemolysis of the red blood cells [4,13-15].
Schubert T T, et al. and other two scientists studied that the enzyme ALP was also markedly elevated in sickle cell disease, but the ratio of serum aspartate aminotransferase and serum alanine aminotransferase enzymes was not altered significantly [13-15].
Figure 3 below showed that there were significant positive Pearson’s Correlation Coefficient between Serum GlutamicOxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) where r=0.929, P=0.000.
They also explained that Sr. AST enzyme was mostly found elevated excessively due to an increased rate of hemolysis of RBCs in sickle cell disease and a significant increase in the level of serum bilirubin was also seen due to the ongoing and increased rate of hemolysis in intrahepatic cholestasis and renal impairments encountered in sickle cell hepatopathy in comparison with the other remaining all diseases [4,13-15]. Thus, the study could be concluded that an increase in SGOT, and SGPT, might be due to the rapid sickling of RBCs in sickle cell disease.
Conclusion
The transaminases SGOT-SGPT enzymes were significantly increased in sickle cell disease so, these two will be other important variables for the diagnosis of sickle cell disease.
Declarations
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding Sources
Nil.
Acknowledgment
I would like to thank all biochemistry departmental staff including teaching and non-teaching, for their timely important advice, help, and encouragement during this study. We also thank the reviewers for their valuable comments and suggestions.
References
- Rees, David C., Thomas N. Williams, and Mark T. Gladwin. "Sickle-cell disease." The Lancet, Vol. 376, No. 9757, 2010, pp. 2018-31.
Google Scholar Crossref - Ballas S.K., "Sickle cell anemia: progress in pathogenesis and treatment." Drugs, Vol. 62, No. 1, 2002, pp. 1143-72.
Crossref - Kakarala, Sri, and Michael Lindberg. "Safety of liver biopsy in acute sickle hepatic crisis." Connecticut medicine, Vol. 68, No. 5, 2004, pp. 277-79.
Google Scholar Crossref - Schubert, Timothy T. "Hepatobiliary system in sickle cell disease." Gastroenterology, Vol. 90, No. 6, 1986, 2013, pp. 1261-68.
Google Scholar Crossref - Gupta, Vikas K., et al. "Status of some hematological and antioxidant parameters in SCA patients of Chhattisgarh Region." Journal of Medical Science and Clinical Research, Vol. 3, No. 7, 2015.
Google Scholar - Andresen, Brian D., and N. V. Bhagavan. "Textbook of clinical chemistry." WB Saunders company, 1986.
Google Scholar - Akuyam, A. S., et al. "Liver function tests profile of sickle cell anaemia patients in steady state of health: Zaria experience." Borno Medical Journal, Vol. 4, 2007, pp. 1-6.
Google Scholar - Richard, S., and H. H. Billett. "Liver function tests in sickle cell disease." Clinical & Laboratory Haematology, Vol. 24, No. 1, 2002, pp. 21-27.
Google Scholar Crossref - Maher, Maha M., and Amany H. Mansour. "Study of chronic hepatopathy in patients with sickle cell disease." Gastroenterology Research, Vol.2, Nol. 6, 2009, p. 338.
Google Scholar Crossref - Gardner, Kate, et al. "How we treat sickle hepatopathy and liver transplantation in adults." Blood, The Journal of the American Society of Hematology, Vol. 123, No. 15, 2014, pp. 2302-07.
Google Scholar Crossref - Brody, Jerome I., William N. Ryan, and Mary A. Haidar. "Serum alkaline phosphatase isoenzymes in sickle cell anemia." Jama, Vol. 232, No. 7, 1975, pp. 738-41.
Google Scholar - Norris, William E. "Acute hepatic sequestration in sickle cell disease." Journal of the National Medical Association, Vol. 96, No. 9, 2004, p. 1235.
Google Scholar - Johnson, CAGE S., et al. "Liver involvement in sickle cell disease." Medicine, Vol. 64, No. 5, 1985, pp. 349-56.
Google Scholar Crossref - Sheehy, T. W. "Sickle cell hepatopathy." Southern medical journal, Vol. 70, No. 5, 1977, pp. 533-38.
Google Scholar Crossref - Stephan, J. L., et al. "Fulminant liver failure in a 12-year-old girl with sickle cell anaemia: favourable outcome after exchange transfusions." European journal of pediatrics, Vol. 154, No. 6, 1995, pp. 469-71.
Google Scholar Crossref