Research Article - International Journal of Medical Research & Health Sciences ( 2025) Volume 14, Issue 2
The Role of Asthma Control Questionnaire for Diagnosis and Assessment of Asthma Control in Children Over 5 Years Old Treated with Fluticasone Propionate and Montelukast
Hasani SH1*, Qorraj H2 and Kuli-Lito GJ32Department of Pharmacology and Toxicology, University Clinical Center, Prishtina, Kosovo
3Department of Pediatric Infectious disease, Clinical University Center “Mother Teresa”, Tirana, Albania
Hasani SH, Department of Pulmonology and Allergy, University Clinical Center, Pediatric Clinic, Prishtina, Kosovo, Email: hasime.qorraj@uni-pr.edu
Received: 13-Oct-2023, Manuscript No. IJMRHS-23-116770; Editor assigned: 16-Oct-2023, Pre QC No. IJMRHS-23-116770 (PQ); Reviewed: 30-Oct-2023, QC No. IJMRHS-23-116770; Revised: 01-Apr-2025, Manuscript No. IJMRHS-23-116770 (R); Published: 08-Apr-2025
Abstract
Purpose: There are many factors that determine the occurrence of asthma, starting with genetic predisposition, host characteristics, and environmental exposure. This makes diagnosing asthma and identifying the severity a problem for pediatricians. Unfortunately, the disease's fundamental mechanism is still not fully understood, yet it includes airway inflammation and airway responsiveness. Since various variables affect the clinical manifestations of asthma, it is seldom viewed as a single illness but rather as a collection of heterogeneous phenotypes that have various causes and prognoses. We compared the two groups treated with prophylaxis: One group with Fluticason propionate (F) and the other with Fluticason Propionate plus Montelukast (F+M), and evaluated the use of Asthma Control Questionnaire (ACQ) for detecting the severity of asthma as well as controlling asthma in children over 5 years old.
Methods: The research is open type, prospective, randomized, comparative, and standardized. It was done as an outpatient procedure in the pediatric clinic. The study involved 65 children, among whom 35 were boys and 30 were girls. Each child had a standard survey filled out in accordance with ISAAC guidelines, and GINA recommendations were used to determine the severity of each child's asthma. After that, the therapy was started and control appointments were made. The children were split into two groups based on the therapy type: The group that received F and the group treated with F+M.
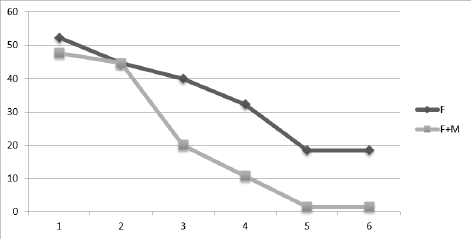
Results: The level of asthma control was evaluated using an Asthma Control Questionnaire-ACQ at each visit for six consecutive months. In the group with F+M, asthma control occurs more quickly and clearly over the course of 6 months: in the third month of treatment, complaints drop to 20%, which results in satisfactory control over asthma symptoms with a further decrease to 1.5% in the sixth month. In contrast, the group with F obtains asthma control later, around the fourth month; by the sixth month, 18.5% of individuals are still receiving treatment.
Conclusions: Prophylactic treatment, which involves long-term inhaler corticosteroid use, is used for medium and severe chronic forms. Unwanted side effects are still a problem and make it difficult to discover alternate long-term therapy options, such as adding Montelukast in place of increasing the dosage of corticosteroids. Adding Montelukast in children with uncontrolled asthma shows earlier improvement compare to those with increased dose of Fluticasone The proper setting of Dg and evaluation of the success of asthma management are made possible by the implementation of the standard ACQ questionnaire.
Keywords
Asthma prophylaxis, Asthma Control Questionnaire (ACQ), Symptoms control, Fluticason, MontelukastIntroduction
One of the most prevalent non-infectious chronic disorders in children is asthma. It is also thought of as a sickness that significantly lowers Quality of Life (QOL) in different aspects which effects on lifestyle, function, group activities, social functioning, mental performance and academic achievement [1,2]. Asthma already affects around 300 million individuals worldwide, and by 2025, it's expected that number will increase by another 100 million. According to the number of years people spend living with a disability, asthma is the 16th leading cause of impairment worldwide and the 28th most prevalent disease. There are regional and socioeconomic variations in the prevalence, severity, and fatality of asthma [3]. There are variations in asthma prevalence and incidence in both children and adults. Although asthma frequently develops in childhood, it is not impossible for it to arise at any other stage in life. It's remarkable that boys experience asthma more frequently than girls before puberty because of smaller airway diameters and that boys experience hospitalizations more frequently than girls, but that this trend entirely reverses in adolescence [4-6]. Asthma is a difficult condition to define because so many things can affect how this ailment manifests. Additionally, the interplay of genetic predisposition, host characteristics, and environmental exposure is frequently cited as its pathogenesis [7]. Air pollution, pollen, mold, hair, and other aeroallergens are examples of environmental variables [8]. Obesity, dietary variables, infections, allergic sensitization, and other host factors are taken into account, while genetic factors as gene loci believed to be related for the predisposition to asthma. The basic mechanism of asthma as a chronic relapsing disease which is regrettably currently unclear but is believed to involve airway inflammation, airway obstruction, and airway responsiveness, is included in the description of asthma as an addition to the many elements we enumerated [9]. Since many variables affect the clinical course of allergies and asthma, it is seldom viewed as a single illness but rather as a collection of different phenotypes with varying etiologies and prognoses [10]. As a result, it is thought that phenotyping asthma patients can greatly aid in the medical treatment of the condition.
Proper phenotyping will ensure tailoring and delivering the right therapy to the right patient at the right time [11]. According to research, the incidence of asthma has increased at the same time that allergies such as hay fever and eczema have become more common [9]. There are numerous theories that have been put up to explain the epidemiological features of asthma, and numerous studies and public discussions have been held on the subject. The "hygiene theory" was put forth in the 1980’s and contends that modernization of living spaces, which lowered the exposure to allergens like home dust, cat hair, and mold, possibly contributed to the rise in asthma and allergy rates [12]. According to Rook's 2003 notion, the respiratory tract and the gut's microbial diversity play a vital role in the immune system's regulation [13].
The diagnosis, determining the severity of the condition, and managing asthma symptoms through preventative care are some of the most difficult aspects of treating children with asthma and good care can be achieved by use of clinical data and simple tests especially when it comes to settings with limited resources [14]. Since preventative treatment necessitates routine inhalation of corticosteroids, adverse effects on the health of children remain to be a global concern and drive for the development of equally effective and less hazardous alternatives [15]. Asthma does not always accompany wheezing, and not all wheezing is caused by asthma, making diagnosis difficult. 50% of children experience wheezing and breathing problems in the first 2 years of life, yet the causes are not always the same. Viral infections are more likely to occur in individuals with underdeveloped immune systems, although genetic predisposition and other environmental factors also play a role. The effects vary from child to child. Then, a variety of disorders and diseases, including Cystic Fibrosis (CF), the presence of foreign substances in the respiratory system, and many others, can cause wheezing in children. These conditions and diseases include infection of the upper respiratory tract (caused by bacteria, viruses, etc.), cystic fibrosis, and many others. The mother's smoking during and after pregnancy, as well as that of the rest of the family, and the family's history of allergy disorders, with an emphasis on the existence of asthma, are undoubtedly environmental variables [16,17]. Wheezing, coughing, shortness of breath, difficulty breathing, and so forth can occur at various ages and can signify a variety of illnesses, including bronchiolitis in infants or a temporary breathing difficulties in children, as well as upper respiratory tract disorders like adenoiditis in young children and sinusitis in older children [19-21]. Asthma among children is therefore a complicated condition, particularly evaluation and therapy. As a result, it necessitates an ongoing patient monitoring not just the significance of the manifestations but also the cause, which can be varied, followed by the medication, which means that we have to modify the doses according to age, weight, and the level of severity of symptoms, as well as monitoring the negative effects of the prophylactic therapy. In several suggestions, the alternative medication Montelukast (antagonist of leukotriene receptors) has been suggested as a way to reduce the side effects of inhaled corticosteroids. An effective treatment strategy for establishing asthma asthma control in pediatric patients and enhancing the quality of life for caregivers is Montelukast as single or in combination with Inhaled Corticosteroids (ICS). This is according to one of the many studies that have been conducted in this direction [22-28].
Materials and Methods
Study design
This study compares the success of controlling asthma with F or with F+M in order to demonstrate the value of employing a standard questionnaire for diagnosing and assessment of asthma for children over 5 years old. The study's open type is standard, prospective, randomized, and comparative. Both hospital and outpatient settings were used for its execution. The parent or legal guardian's permission was first required before the child could be included in the study. The study involved 65 children, among whom 35 are boys and 30 are girls. The following laboratory tests, an anamnesis, and objective evaluation were done: Spirometry, sedimentation rate, antistreptolisin test, prick test, total IgE, and parasite screening of feces.
Evaluation of treatment efficacy
Each child completed an ISAAC-required standard survey, and the severity of their asthma was assessed in accordance with GINA guidelines. After that, the therapy was started and control appointments were made. The children were split into two groups based on the therapy type: The group that was receiving therapy F and the other group receiving F+M. For six continuous months, each child received a monthly visit. The asthma control questionnaire (Asthma Control Questionnaire-ACQ) was utilized during these sessions to re-evaluate the patient's health, the patient's reaction to the prescribed preventive medication, and the patient's assessment of risk factors. The "Step-Up" and "Step-Down" method of treatment was used throughout these encounters. Children between the ages of 5 and 17 who had mild to moderate persistent asthma symptoms and had not previously received preventive medication were the study's subjects. Children with intermittent or severe asthma, children receiving preventative treatment for asthma, children with other lung disorders and with other associated diseases of other biological systems-all of these children weren't included in the study.
Research plan: Opening the card bearing the serial number, getting parental or guardian approval, and completing the standard ISAAC questionnaire are all part of the research plan. Entering broad information, such as demographic details, life anamnesis, family information, socio-epidemiological information, and hygienic-sanitary information. Spirometry and lab testing are scheduled. Every 4 weeks, the occurrence of risk factors and the occurrence of symptoms were re-evaluated. It is chosen how to carry on the therapy at each visit. The Excel record was made after the data had been gathered and six months had passed since the last patient.
Results and Discussion
According to the Children's Wheezing and Asthma Guide NHS Luton Clinical Commissioning group, the identification of a distinctive pattern of breathing symptoms, signs, and test findings, as well as the absence of any other plausible explanations, serve as the foundation for the diagnosis of children with asthma [29]. A clinical assessment for the possibility of asthma should be done in combination with the testing. Since it might be challenging to diagnose asthma in young children, the term "suspected asthma" can be applied and the guardian or parent is given the relevant information [30]. Asthma in children can only be confirmed when the child can undergo objective tests. The presence of these symptoms-wheezing, coughing, breathing difficulties, shortness of breath, and tightness in the chest-guides to a diagnosis of asthma in children aged 5 to 16 [31].
The most common symptom was cough, which was observed in 98.5% of cases along with a cold, and 80% of cases included dry coughing at night time or a cough along with a common cold or illness.[32]
Wheezing persisted in 46.2% of instances despite the absence of cold symptoms.
Wheeze and chest tightness were present in 89.2% of cases, and 70.7% of wheezing episodes lasted 3 to 9 days. 50.8% of respondents experienced between four and twelve wheeze episodes in the previous year, and the same proportion of children experienced sleep disturbance as a result of wheezing, compared to 61.5% of those who wheezed as a result of physical activity. Over half (52.3%) of patients reported wheezing that was so acute that they could only speak one or two sentences at a time in between breaths [33].
During and after exercise, 61.5% of our cases experienced wheezy chest. 93.8% of parents claim that their child doesn't have asthma, which demonstrates a significant degree of denial despite the fact that 95.4% of them have had asthma therapy [34].
Chest tightening and cough with secretion were both present in 44.6% of cases, depending on the patient's ability to explain these symptoms as well as their capacity to expectorate when coughing [35].
After assessing the severity of our patients' asthma, we separated them into two groups based on the type of treatment they received: one group received Fluticasone, and the other received Fluticasone+Montelukast.
For six consecutive months, all patients were reviewed once a month to determine whether or not their asthma was under control. At each appointment, the primary symptoms were assessed using a standardized questionnaire [36].
The prophylactic treatment of persistent asthma in children is currently dominated by corticosteroids like Fluticasone propinate, but due to the risks associated with long-term steroid use, the stigma associated with using a pump, particularly in our population, as well as poor compliance or improper use techniques, there is a necessity for a substitute therapy, which in our study is the addition of Montelukast as add on therapy instead of increasing the dose of inhaled corticosteroids.
Table 1 contains core questionnaires which enable us to describe the prevalence and severity of asthma in our chosen population group. Those questionnaires allow us to investigate possible aetiological factors: Genetic, lifestyle, environmental and care factors.
| Questions | Answers | Boys | Girls | In total | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| In the previous 12 months, did your child have chest wheezing? And how long did it last? |
1-3 days | 10 | 28.6 | 4 | 13.3 | 14 | 21.5 |
| 3-6 days | 14 | 40 | 13 | 43.3 | 27 | 41.5 | |
| 6-9 days | 9 | 25.7 | 10 | 33.3 | 19 | 29.2 | |
| 10-14 days | 2 | 5.7 | 2 | 6.7 | 4 | 6.2 | |
| >14 days | 0 | 0 | 1 | 3.3 | 1 | 1.5 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| How many wheezing episodes did your child have in the last 12 months? | 1-3 times | 13 | 37.1 | 12 | 40 | 25 | 38.5 |
| 4-12 times | 18 | 51.4 | 15 | 50 | 33 | 50.8 | |
| >12 | 4 | 11.4 | 3 | 10 | 7 | 10.8 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| x2-test | P=0.07 | ||||||
| How frequently did your child have sleep issues | Never | 8 | 22.9 | 5 | 16.7 | 13 | 20 |
| >one night a week | 16 | 45.7 | 17 | 56.7 | 33 | 50.8 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| X2-test | P=0.816 | ||||||
| Over the previous year, Was wheezing interfering with your child's speech? | Yes | 18 | 51.4 | 16 | 53.3 | 34 | 52.3 |
| No | 17 | 48.6 | 14 | 46.7 | 31 | 47.7 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| X2-test | P=0.878 | ||||||
| Has your child ever wheezed during or after physical activity? | Yes | 19 | 54.3 | 21 | 70 | 40 | 61.5 |
| No | 16 | 45.7 | 9 | 30 | 25 | 38.5 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| X2-test | P=0.297 | ||||||
| Has your child experienced a nighttime dry cough or a cough that was associated with an infection in the past 12 months? | Yes | 30 | 85.7 | 22 | 73.3 | 52 | 80 |
| No | 5 | 14.3 | 8 | 26.7 | 13 | 20 | |
| X2-test | P=0.350 | ||||||
| Does the child typically cough when they have a cold? | Yes | 35 | 100 | 29 | 96.7 | 64 | 98.5 |
| No | - | - | 1 | 3.3 | 1 | 1.5 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| Fisher test | P=0.461 | ||||||
| When not contagious, does your child have a habit of coughing? | Yes | 18 | 51.4 | 12 | 40 | 30 | 46.2 |
| No | 17 | 48.6 | 18 | 60 | 35 | 53.8 | |
| X2-test | P=0.501 | ||||||
| Has your youngster wheezed in the last 12 months together with the flu or a cough? | Yes | 31 | 88.6 | 27 | 90 | 58 | 89.2 |
| No | 4 | 11.4 | 3 | 10 | 7 | 10.8 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| Fisher test | P=0.999 | ||||||
| Has the youngster wheezed recently | Yes | 18 | 51.5 | 16 | 53.3 | 34 | 52.3 |
| No | 17 | 48.6 | 14 | 46.7 | 31 | 47.7 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| X2-test | P=0.878 | ||||||
| Does your child have any prior asthma? | Yes | 2 | 5.7 | 2 | 6.7 | 4 | 6.2 |
| No | 33 | 94.3 | 28 | 93.3 | 61 | 93.8 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| Fisher test | P=1.000 | ||||||
| Has your child had treatment in the previous 12 months for asthma or wheezing? | Yes | 35 | 100 | 27 | 90 | 62 | 95.4 |
| No | - | - | 3 | 10 | 3 | 4.6 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| What medications did your child take? | Salbutamol | 4 | 11.4 | 9 | 33.3 | 13 | 21 |
| Steroids+Salbutamol | 16 | 45.7 | 6 | 22.2 | 22 | 35.5 | |
| Antibiotics+Salbutamol | 12 | 34.3 | 12 | 44.4 | 24 | 38.7 | |
| In the last 12 months did your child had chest tightnes or cough up sputum without symptoms of cold? | Yes | 21 | 60 | 8 | 26.7 | 29 | 44.6 |
| No | 14 | 40 | 22 | 73.3 | 36 | 55.4 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| X2-test | P=0.014 | ||||||
| In the last 12 months, how many times have had a cold or flu? | Never | 3 | 8.6 | 2 | 6.7 | 5 | 7.7 |
| 1-3 times | 14 | 40 | 11 | 36.7 | 25 | 38.5 | |
| 4-6 times | 14 | 40 | 13 | 43.3 | 27 | 41.5 | |
| ≥ 7 times | 4 | 11.4 | 4 | 13.3 | 8 | 12.3 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| X2-test | P=0.975 | ||||||
| Spirometry | FEV1 normal | 5 | 14.3 | 7 | 23.3 | 12 | 18.5 |
| FEV1 low | 20 | 57.1 | 14 | 46.7 | 34 | 52.3 | |
| No spirometry | 10 | 28.6 | 9 | 30 | 19 | 29.2 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| X2-test | P=0.501 | ||||||
| Total IgE | IgE tot normal | 14 | 40 | 11 | 36.7 | 25 | 38.5 |
| IgE tot high | 21 | 60 | 19 | 63.3 | 40 | 61.5 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| X2-test | P=0.984 | ||||||
| ASO | Normal | 31 | 88.6 | 21 | 70 | 52 | 80 |
| High | 4 | 11.4 | 9 | 30 | 13 | 20 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| Fisher test | P=0.117 | ||||||
| Prick test | 1 Allergy | 3 | 8.6 | 10 | 33.3 | 13 | 20 |
| 2 Allergy | 14 | 40 | 4 | 13.3 | 18 | 27.7 | |
| 3 Allergy | 5 | 14.3 | 4 | 13.3 | 9 | 13.8 | |
| 4 Allergy | 13 | 37.1 | 12 | 40 | 25 | 38.5 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| Intestinal parasitosis | Positive | 3 | 8.6 | 6 | 20 | 9 | 13.8 |
| Negative | 32 | 91.4 | 24 | 80 | 56 | 86.2 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| Nose eosinophylia | Positive | 15 | 42.9 | 13 | 43.3 | 28 | 43.1 |
| Negative | 20 | 57.1 | 13 | 56.7 | 37 | 56.9 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| Fisher test | P=0.969 | ||||||
| Asthma gravity before therapy | Mild | 6 | 17.1 | 7 | 23.3 | 13 | 20 |
| Medium | 29 | 82.9 | 23 | 76.7 | 52 | 80 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
| X2-test | P=0.755 | ||||||
| Starting treatment with F or F+M | Fluticasone (F) | 19 | 54.3 | 14 | 46.7 | 33 | 50.8 |
| Fluticasone+Montelukast (F+M) | 16 | 45.7 | 16 | 53.3 | 32 | 49.2 | |
| In total | 35 | 100 | 30 | 100 | 65 | 100 | |
|
X2-test |
P=0.716 |
||||||
Table 1. Contains core questionnaires which enable us to describe the prevalence and severity of asthma in our chosen population group.
In Table 1 we find that in the question. How many wheezing episodes did your child have in the last 12 months? There is difference between girls and boys and results are in the margin of statistical significance P=0.07 and also in question Did your child have chest tightness or cough up sputum without symptoms of cold the result just very slightly missed the significance level P= 0.014.
The following primary asthma control indicators were tracked and recorded over the course of a six-month follow-up period: Cough, wheezing, sleeping issues brought on by coughing, the requirement for quick relief medication (Salbutamol), physical activity restriction, and spirometry values: FEV1 and FVC. The third month was a period when improvements occurred for nearly all of those markers for roughly 50%. Regarding the difference between the two sexes in the main indicators of asthma control there was not sufficiently strong evidence to reject the null hypothesis and conclude that the groups are different (Table 2).
| Cough | Wheez>2 | Diff. brea | Sleep dis. | Salbutamol | Activity | FEV1 | FEV1+FVC | |
|---|---|---|---|---|---|---|---|---|
| Month 1 | 96.9 | 90.8 | 90.8 | 92.3 | 95.4 | 98.5 | 46.2 | 44.6 |
| Month 2 | 70.8 | 33.8 | 18.5 | 32.3 | 70.8 | 43.1 | 38.5 | 30.8 |
| Month 3 | 46.2 | 12.3 | 7.7 | 12.3 | 60 | 33.8 | 12.3 | 10.8 |
| Month 4 | 24.6 | 3.1 | 3.1 | 7.7 | 43.1 | 21.5 | 10.8 | 9.2 |
| Month 5 | 12.3 | 7.7 | 6.2 | 4.6 | 23.1 | 16.9 | 10.8 | 10.8 |
| Month 6 | 6.2 | 4.6 | 4.6 | 6.2 | 12.3 | 9.2 | 3.1 | 3.1 |
| X2-test | P=0,521 | P=0,474 | P=0,215 | P=0,215 | P=0,817 | P=0,301 | P=0-,962 | P=0,987 |
Table 2. Main indicators of asthma control.
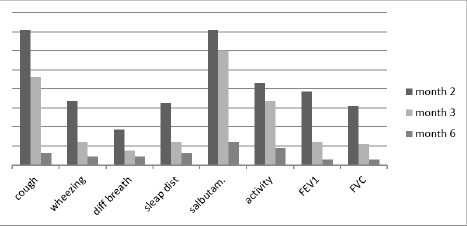
While cough and the need for quick relief medication improved in month IV, wheezing and chest tightness, difficulty breathing, sleeping disturbance due to cough, and physical activity limitation improved even earlier in the second month after starting preventive treatment.
When we compare the effectiveness of treatment between two groups of children treated with F and F+M, we find that the need for fast reliever and spirometry levels of FEV1 and FVC were similarly improved in both groups, whereas cough>twice a week, wheezing>twice a week, difficulty breathing, and sleep disturbance due to cough were much better and earlier improved in the group of children treated with F+M (Figure 1).
Figure 1. Improvement of asthma symptoms through 6 months.
A chi-square test is performed between male and female group and also between two groups with different treatment. The results were not statistically significant.
We can observe that for the group with F+M, asthma management occurs more quickly and dramatically over the course of 6 months: in the third month, asthma symptoms fall to 20%, and they continue to decline to 1.5% in the sixth month. In contrast, the group receiving only Fluticasone treatment reaches asthma control later, during the fourth month, and by the sixth month, 18.5% of cases are still receiving treatment (Figure 2).
Figure 2. Prophylactic therapy through 6 month.
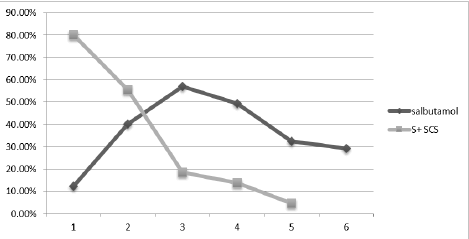
Additionally, we looked at the occurrence of asthma attacks over the course of six months and came to the conclusion that children receiving preventative therapy gradually reduced their use of S+SCS, falling to 20% in the third month and 2% in the sixth. In the third month, as the administration of steroids declines, the usage of Salbutamol (S) increases, indicating greater control and in accordance with guidelines for managing asthma attacks (Figure 3).
Figure 3. Asthma attack management over the months with prophylaxy.
Conclusion
70.7% of 65 children aged 5 and older had wheezing that persisted for 3 to 9 days. 50.8% of those surveyed reported four to twelve wheeze episodes in the previous year, and the same percentage of children experienced sleep disturbances brought on by wheezing. Very acute wheeze and speech pauses were present in 52.3%. 61.5% of those who exercised reported wheezing. 80% of those surveyed reported a night time dry cough or a cough that was triggered by a common cold or infection. 98.5% of people who catch a cold had a cough. 46.2% of people reported a cough without having common cold. Wheezing and tightness in the chest were present in 89.2% of cases with flu or cough. In addition to the flu or cough, 52.3% also experienced wheezing and chest discomfort. 93.8% of parents affirm that their child is asthma-free. 95.4% have gotten asthma therapy. 95.2% of patients got salbutamol, steroids, and antibiotics. 81.5% had a whooping cough when they catch a cold. 52.3% had reduced FEV 1, 61.5% had high IgE, and 41.5% had four to six influenza or cold episodes each year. 80% had normal ASTO levels. The positive prick test resulted from 83.1% of the population. Tests for intestinal parasitosis were negative in 86.2%. 43.1% of nasal samples had eosinophils. Following the preventative therapy's: Symptoms of breathing difficulty in the fifth and sixth months: Zero percent among the group of patients treated with F+M whereas among the group treated with F this symptom was present 12% in the fifth month of treatment, and 9% in the final month of treatment. In the fifth month, the cough-related symptoms of sleep disturbance decreased by 0% for the F+M treated group while it persisted in 9.1% of the F treated group. Both the requirement for salbutamol and the restriction on physical activity improved virtually equally for the two groups. The improvement in FEV1 and FVC were identical for both groups. Children who use preventative medications experience fewer asthma attacks, use of SCS declines, and usage of salbutamol rises in response to guidelines for the management of asthma episodes.
References
- Valizadeh L, et al. Effect of education and controlling Asthma triggers on quality of life among adolescents with asthma: A randomized clinical trial. Journal of Mazandaran University of Medical Sciences, Vol. 22, No. 98, 2013, pp. 49-57.
- Kiotseridis H, et al. Adherence and quality of life in adults and children during 3-years of SLIT treatment with Grazax-a real life study. NPJ Primary Care Respiratory Medicine, Vol. 28, No. 1, 2018, pp. 4.
[Crossref] [Google Scholar] [PubMed]
- Asher MI, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. The Lancet, Vol. 368, No. 9537, 2006, pp. 733-743.
[Crossref] [Google Scholar] [PubMed]
- To T, et al. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health, Vol. 12, 2012, pp. 1-8.
[Crossref] [Google Scholar] [PubMed]
- Chowdhury NU, et al. Sex and gender in asthma. European Respiratory Review, Vol. 30, No. 162, 2021, pp. 210067.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, et al. Sex difference in hospitalization due to asthma in relation to age. Journal of Clinical Epidemiology, Vol. 56, No. 2, 2003, pp. 180-187.
[Crossref] [Google Scholar] [PubMed]
- Louisias M, et al. The effects of the environment on asthma disease activity. Immunology and Allergy Clinics, Vol. 39, No. 2, 2019, pp. 163-175.
[Crossref] [Google Scholar] [PubMed]
- Dick S, et al. Associations between environmental exposures and asthma control and exacerbations in young children: A systematic review. BMJ Open,Vol. 4, No. 2, 2014, pp. 1-7.
[Crossref] [Google Scholar] [PubMed]
- Eder W, Ege MJ and von Mutius E. The asthma epidemic. The New England Journal of Medicine, Vol. 355, No. 21, 2006, pp. 2226–2235.
[Crossref] [Google Scholar] [PubMed]
- Pavord ID, et al. After asthma: Redefining airways diseases. The Lancet, Vol. 391, No. 10118, 2018, pp. 350-400.
[Crossref] [Google Scholar] [PubMed]
- Schatz M, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. Journal of Allergy and Clinical Immunology, Vol. 133, No. 6, 2014, pp. 1549-1556.
[Crossref] [Google Scholar] [PubMed]
- Dharmage SC, Perret JL, and Custovic A. Epidemiology of asthma in children and adults. Frontiers in Pediatrics, Vol. 7, 2019, pp. 246.
[Crossref] [Google Scholar] [PubMed]
- Bloomfield SF, et al. Time to abandon the hygiene hypothesis: New perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspectives in Public Health, Vol. 136, No. 4, 2016, pp. 213-224.
[Crossref] [Google Scholar] [PubMed]
- Serugendo AN, et al. Evaluation of asthma control using Global Initiative for Asthma criteria and the Asthma Control Test in Uganda. The International Journal of Tuberculosis and Lung Disease, Vol. 18, No. 3, 2014, pp. 371-376.
[Crossref] [Google Scholar] [PubMed]
- Sawicki G, Haver K and Redding G. Asthma in children younger than 12 years: Management of persistent asthma with controller therapies. UpToDate. Waltham.
- Grigg J, Ducharme FM. Asthma in the preschool age child. Kendig's Disorders of the Respiratory Tract in Children, 2019, pp. 677-685.
- Harju M, et al. Parental smoking and cessation during pregnancy and the risk of childhood asthma. BMC Public Health, Vol. 16, 2016, pp. 1-7.
[Crossref] [Google Scholar] [PubMed]
- Fedele G, et al. Analysis of the immune response in infants hospitalized with viral bronchiolitis shows different Th1/Th2 profiles associated with respiratory syncytial virus and human rhinovirus. Pediatric Allergy and Immunology, Vol. 29, No. 5, 2018, 555-557.
[Crossref] [Google Scholar] [PubMed]
- Midulla F, et al. How respiratory syncytial virus genotypes influence the clinical course in infants hospitalized for bronchiolitis. The Journal of Infectious Diseases, Vol. 219, No. 4, 2019, 526-534.
[Crossref] [Google Scholar] [PubMed]
- Rubner FJ, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. Journal of Allergy and Clinical Immunology, Vol. 139, No. 2, 2017, 501-507.
[Crossref] [Google Scholar] [PubMed]
- Dumas O, et al. Severe bronchiolitis profiles and risk of asthma development in Finnish children. Journal of Allergy and Clinical Immunology, Vol. 149, No. 4, 2022, pp. 1281-1285.
[Crossref] [Google Scholar] [PubMed]
- Berube D, et al. Effectiveness of montelukast administered as monotherapy or in combination with inhaled corticosteroid in pediatric patients with uncontrolled asthma: A prospective cohort study. Allergy, Asthma and Clinical Immunology, Vol. 10, 2014, pp. 1-2.
[Crossref] [Google Scholar] [PubMed]
- Nagao M, et al. Early control treatment with montelukast in preschool children with asthma: A randomized controlled trial. Allergology International, Vol. 67, No. 1, 2018, pp. 72-78.
[Crossref] [Google Scholar] [PubMed]
- Hon KL, Leung TF and Leung AK. Clinical effectiveness and safety of montelukast in asthma. What are the conclusions from clinical trials and meta-analyses?. Drug Design, Development and Therapy, Vol. 8, 2014, pp. 839-850.
[Crossref] [Google Scholar] [PubMed]
- Chauhan BF, et al. Addition of antiâ?leukotriene agents to inhaled corticosteroids for adults and adolescents with persistent asthma. Cochrane Database of Systematic Reviews, Vol. 3, No. 3, 2017, pp. 1-168.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, et al. lncRNA PCGEM1 strengthens anti-inflammatory and lung protective effects of montelukast sodium in children with cough-variant asthma. Brazilian Journal of Medical and Biological Research, Vol. 53, 2020, pp. e9271
[Crossref] [Google Scholar] [PubMed]
- Brodlie M, et al. Leukotriene receptor antagonists as maintenance and intermittent therapy for episodic viral wheeze in children. The Cochrane Database of Systematic Reviews, Vol. 2015, No. 10, 2015, pp. CD008202.
[Crossref] [Google Scholar] [PubMed]
- Magwenzi P, et al. Challenges in the diagnosis of asthma in children, what are the solutions? A scoping review of 3 countries in sub Saharan Africa. Respiratory Research, Vol. 23, No. 1, 2022, pp. 254.
[Crossref] [Google Scholar] [PubMed]
- Asher ME, et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. European Respiratory Journal, Vol. 8, No. 3, 1995, pp. 483-491.
[Crossref] [Google Scholar] [PubMed]
- Lindenhofer M, et al. Wheeze and cough measurements at night in children with respiratory symptoms. BMC Pediatrics, Vol. 20, 2020, pp. 1-9.
[Crossref] [Google Scholar] [PubMed]
- Kim SY, Sim S and Choi HG. Active and passive smoking impacts on asthma with quantitative and temporal relations: A Korean Community Health Survey. Scientific Reports, Vol. 8, No. 1, 2018, pp. 8614.
[Crossref] [Google Scholar] [PubMed]
- Snehalatha G, et al. A study on correlation of serum IgE levels with diagnosis and severity of asthma in children. International Journal of Contemporary Pediatrics, Vol. 5, No. 6, 2018, pp. 2240-2243.
- Del Carmen Trojavchich M, et al. Relationship between group A betaâ?hemolytic streptococcal tonsillopharyngitis and asthma. Pediatric Allergy and Immunology, Vol. 14, No. 1, 2003, pp. 50-54.
[Crossref] [Google Scholar] [PubMed]
- Pereira MU, et al. Nonatopic asthma is associated with helminth infections and bronchiolitis in poor children. European Respiratory Journal, Vol. 29, No. 6, 2007, pp. 1154-1160.
[Crossref] [Google Scholar] [PubMed]
- Murayama N and Murayama K. Nasal discharge eosinophils in childhood asthma patients as a predictive factor for persistent asthma. Mediators of Inflammation. Vol. 2018, No. 1, 2018. pp. 2563978.
[Crossref] [Google Scholar] [PubMed]
- Holgate ST. Mechanisms of asthma and implications for its prevention and treatment: A personal journey. Allergy, Asthma and Immunology Research, Vol. 5, No. 6, 2013, pp.343-347.
[Crossref] [Google Scholar] [PubMed]