Research - International Journal of Medical Research & Health Sciences ( 2021) Volume 10, Issue 2
Usefulness of Measurement of Soluble Interleukin-2 Receptor (sIL-2R) and Interleukin 6 to Monitor Efficacy of Specific Immunotherapy in Eosinophilic Esophagitis
Alicia Armentia1, Angel San Miguel Rodríguez2*, Blanca Martin-Armentia2, Sara Martin-Armentia3, Manuel Gonzalez-Sagrado2, Angel San Miguel Hernandez4, Rosa Conde2, Maria del Carmen Lozano5 and Irene Iglesias Peinado62Research Service, Hospital Universitario Río Hortega, Valladolid, Spain
3Primare care, Paedriatics, Delicias, Valladolid, Spain
4Clinical Analysis Service, Hospital Universitario Río Hortega, Spain
5Department of Pharmacy, Alfonso X el Sabio University, Madrid, Spain
6Department of Pharmacy, Complutense University, Madrid, Spain
Angel San Miguel Rodríguez, Research Service, Hospital Universitario Río Hortega, Valladolid, Spain, Email: asanmi@saludcastillayleon.es
Received: 30-Dec-2020 Accepted Date: Feb 11, 2021 ; Published: 18-Feb-2021
Abstract
Background: Eosinophilic esophagitis is considered a hypersensitivity reaction. We have successfully treated patients who suffered from eosinophilic esophagitis with Allergen Specific Immunotherapy (AIT). Monitoring this efficacy has been carried out with clinical parameters and serial endoscopies, but it would be useful to find a serological marker with a good response to the AIT that would obviate them. Soluble Interleukin-2 Receptor (sIL-2R) can be useful for monitoring many active eosinophilic inflammatory diseases (autoimmune, neoplastic, and infectious), but it has not been measured in Eosinophilic Esophagitis (EoE). IL-6 has a great inflammatory activity more associated with rheumatoid arthritis but could measure the lymphocytic and fibrotic response that occurs in the remodeling of the mucosa in this pathology. Objective: We carried out measurements of sIL-2Ry IL-6 after 3 years of AIT in patients with EoE and two years of suspension of this treatment without relapse of disease, to evaluate the usefulness of this measurement as objective markers of improvement of the esophagitis. Methods: One hundred and twenty-nine patients with EoE were tested for environmental and food allergens. CRD, histological and botanical analysis was performed. Clinical scores and endoscopic biopsies were performed every six months for five years. Fifteen healthy patients and 34 asthmatics due to pollen were included as control groups. CRD-directed Allergen Immunotherapy (AIT) was administered in 91 EoE patients and conventional treatment (proton pump inhibitors, empiric diet, corticosteroids) in the rest of the patients (n=38). Sera of all patients were collected before de therapy and after the suppression of the treatment. Randomized blind analysis of IL-2R and IL-6 was performed in all samples of treated (AIT/conventional) and controls subjects and correlated with clinical and endoscopic findings. Results: Higher sIL-2Rlevels were measured in patients with EoE for pollen asthma and higher in both processes than in healthy patients (p<0.0001). In patients treated with AIT, a marked decrease in sIL-2R was observed, significantly higher than in patients with conventional therapy (p<0.0001). This improvement was significantly correlated with clinical and endoscopic findings of good evolution of the disease (p<0.001). The measure of IL-6 was not useful for monitoring the effect of AIT. Conclusion: Measurement of sIL-2R can be useful in monitoring efficacy of specific immunotherapy in EoE as can be used also as a marker of the activity of the disease, but it is not valid as a diagnostic test, while IL-6 is not valid neither for monitoring nor as a diagnostic test.
Keywords
sILR2, IL-6, Eosinophilic esophagitis, Allergen immunotherapy, Component resolved diagnosis
Introduction
In the last years, we have successfully treated patients suffered from Eosinophilic Esophagitis (EoE) with Allergen Specific Immunotherapy (AIT) and diet guided by Component-Resolved Diagnosis (CRD) [1,2]. The monitoring of this efficacy has been carried out with clinical parameters and serial endoscopies, but it would be useful to find a serological marker with a good response to the AIT that would obviate them.
Eosinophils appear in large numbers at inflammation sites in response to certain parasitic infections [3]. These leukocytes, when mature, reside mostly in tissues, but about 1% of the eosinophil population circulates in the blood. Activated eosinophils release highly basic proteins into the surrounding tissue. The granular proteins, which can kill parasites and some mammalian cells, might cause the tissue damage associated with asthma and other inflammatory diseases [4]. Eosinophil activation accompanies a wide range of inflammatory diseases, including bronchial asthma and eosinophilic esophagitis [4-6].
Among the interleukins, IL-6 has proven a useful monitor for many active inflammatory diseases [7]. sIL-2R concentrations in plasma and certain other body fluids increase during inflammatory reactions [8]. sIL-2R interleukin plays a key role in the regulation of the immune response. The binding of IL-2 to its receptor (IL-2R), on the surface of Tlymphocytes, triggers a series of signaling events between cells that produce the activation and proliferation of resting T-cells and, ultimately, the generation of cytotoxic T-cells, suppressors, and auxiliaries, that act as intermediaries in the immune reactions [8].
Eosinophilic Esophagitis (EoE) is characterized by esophageal dysfunction and, histologically, by eosinophilic inflammation. Conventional treatment (Proton Pump Inhibitors (PPIs), corticosteroids, empiric diet, dilations) did not resolve the symptoms in all patients. Environmental allergies to substances such as dust mites, animals, pollen, and molds can play a role in EoE. Allergy testing for these common environmental allergies is often part of the EoE evaluation [4]. For some patients, it may seem like their EoE is worse during pollen seasons [1,2,6,9].
Six years ago, 129 patients with EoE were tested for environmental and food allergens [9]. Component Resolved Diagnosis (CRD), histological and botanical analysis was performed. Microscopic examination of esophageal biopsies of 129 adult patients with EoE, 82 of them with seasonal exacerbation, and 100 controls, with gastroesophageal reflux without eosinophilic infiltrate, were made to verify the presence of callose (polysaccharide abundant in pollen tubes but absent in animal tissues) in the esophagus.
CRD detected pollen allergens in 87.6% of patients with EoE and Lipid Transfer Proteins (LTPs) of common Mediterranean foods such as peach, hazelnuts, walnuts, and wheat in 19.4%. Callose from pollen tubes was found in 65.6% of biopsies. Alteration of the mucosal barrier in EoE might cause the penetration of pollen grains, spores, and other food particles into the esophageal tissues. Eosinophils seemed to act as if they were responding to parasitic infections [2]. In EoE patients, histological studies searching for the intrusion to plant foods and pollen, and specific-guided diet and immunotherapy after plant structures detection in biopsies were useful to identify the possible cause of EoE [1,2].
We began CRD guided specific immunotherapy in these patients. After the CRD-guided elimination diet and/or AIT, 101 (78.3%) EoE patients showed significant clinical improvement (p<0.017), and 97 (75.2%) were discharged (negative biopsy, no symptoms, no medication) without relapse. AIT-treated patients had better outcomes (odds ratio 177.3, 95% CI 16.2-1939.0) than the patients with conventional treatment.
We have to keep refrigerated the sera of the patients before the treatment, at the moment of the first diagnosis, and now we compared the levels of IL-6 and IL-2R in 36 patients sera after 3 years of immunotherapy and 2 years of suppression of AIT due to the resolution of this disease (negative clinical and biopsy findings) with 38 samples of patients that were treated with conventional therapy in the same period, that continue with symptoms.
The aim was to obtain an objective marker of improvement of the EoE that may obviate or reduce the need for endoscopic biopsies.
Materials and Methods
One hundred and twenty-nine patients with EoE were tested for environmental and food allergens. CRD, histological and botanical analysis was performed. Clinical scores and endoscopic biopsies were performed every six months for five years. Fifteen healthy patients and 34 asthmatics due to pollen were included as control groups. These patients were provided with sufficient information to decide whether to receive usual therapy (oral corticosteroids, proton pump inhibitors, 6-food diet) or CRD-guided diet and AIT. The patient information sheet and informed consent form, and further informed consent for the histological biopsy study, were approved by the University Hospital Rio Hortega Ethics Committee.
CRD-directed Allergen Immunotherapy (AIT) was administered in 91 EoE patients and conventional treatment (proton pump inhibitors, empiric diet, corticosteroids) in the rest of the patients (38). Sera of all patients were collected before de therapy and after the suppression of the treatment.
We compared now the levels of IL-6 and sIL-2R in 36 patient´s sera after 3 years of immunotherapy and 2 years of suppression of AIT due to the resolution of this disease (negative clinical and biopsy findings) with the levels of the same IL-6 and IL-2R in the 38 patients that followed conventional treatment.
Randomized blind analysis of IL-6 and sIL-2R was performed in all samples of treated (AIT/conventional) and controls subjects and correlated with clinical and endoscopic findings.
The serum samples were collected, after blood extraction and subsequent centrifugation, in polystyrene tubes for storage, instead of glass tubes, to avoid the decrease of IL values. The samples were frozen in a library at -20ºC, until the end of the study.
The method to measure IL-6 and sIL-2R in serum was Immulite 2000 ECP Siemens, German, a solid-phase, two-site chemiluminescent immunometric assay, which was carried out following the manufacturer’s recommendations. The expected values of sIL-2R in our laboratory in healthy individuals was 333 U/ml with a reference range of 95% de 158 a 623 U/ml. We also followed the National Committee for Clinical Laboratory Standards Procedures for the collection of diagnostic blood specimens [10].
The measurement technique of sIL-2R with Immulite 2000, presents a calibration range up to 200 ng/mL and analytical sensitivity of 0.2 ng/mL.
Statistical Analysis
IL-6 and IL-2R levels were described as mean ± Standard Deviation (SD). At baseline, ANOVA with Bonferroni posthoc test was performed to detect differences between groups. The no parametric Wilcoxon test for paired samples was used to compare pre and post-AIT means. To determine de best cut-off values to detect EoE, a Receiving Operating Characteristic (ROC) curve analysis was performed, and sensibility, specificity, and predictive values were calculated. For all tests, a significance level was established in p<0.05.
Results
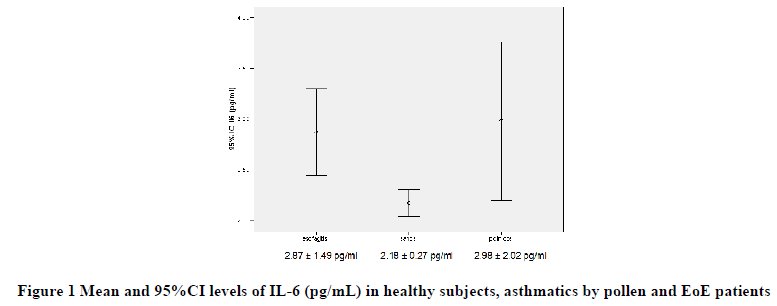
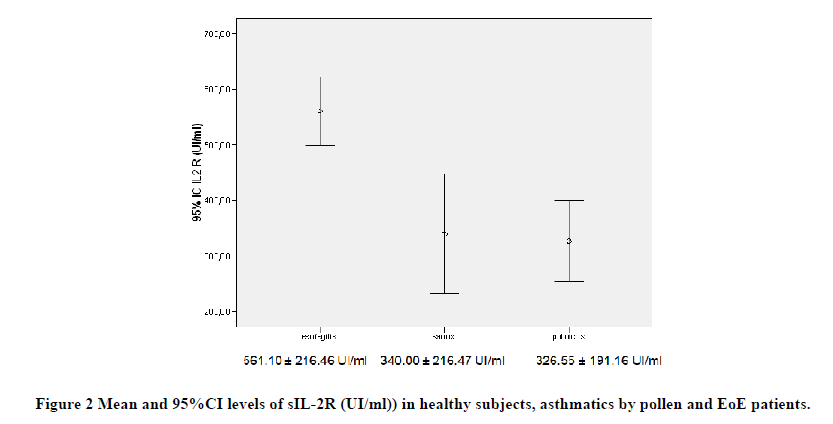
Before treatment, mean IL-6 and sIL-2R levels were higher in patients with EoE (2.87 ± 1.49 pg/ml and 561.10 ± 216.46 UI/ml) in relation to asthmatic groups (2.98 ± 2.02 pg/ml and 326.55 ± 191.16 UI/ml) and healthy controls (2.18 ± 0.27 pg/ml and 340.00 ± 216.47 UI/ml), respectively, with a significant intergroup difference (p<0.0001).
Besides, the IL-6 and sIL-2R levels were higher in patients with EoE than both in asthmatics due to pollen (p<0.001) and in healthy controls (p<0.001), while no differences were detected between asthmatic and healthy subjects (Figures 1 and 2).
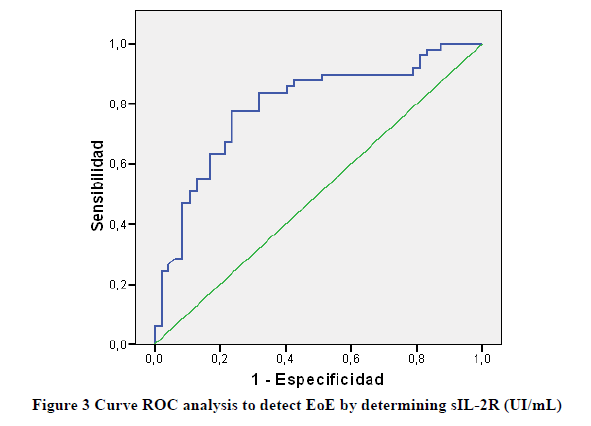
To detect EoE at baseline, sIL-2R values showed an area under the curve of 0.92 (CI 95% 0.700-0.884) (Figure 3).
The best cut-off was established in 460 ng/ml, with a sensitivity of 77.6% and a specificity of 76.6% (Table 1).
| sIL-2R=460 UI/mL | sIL-2R=394 UI/mL | |||
|---|---|---|---|---|
| Value (%) | CI 95% | Value (%) | CI 95% | |
| Sensitivity | 77.6 | (64.9-90.2) | 83.7 | (72.3-95.0) |
| Specificity | 76.6 | (63.4-89.8) | 68.1 | (53.7-82.5) |
| Positive predictive value | 77.6 | (64.8-90.2) | 73.2 | (60.7-85.7) |
| Negative predictive value | 76.6 | (63.4-89.8) | 80.0 | (66.4-93.7) |
| Positive likelihood ratio | 3.3 | (1.9-5.7 ) | 2.62 | (1.7-4.1) |
| Negative likelihood ratio | 0.3 | (0.2-0.5) | 0.24 | (0.12-0.47) |
Table 1: Diagnostic test study of ILR2 to detect EoE.
The best cut-off was established in 394 ng/ml, with a sensitivity of 83.7% and a specificity of 68.1% (Table 1).
Favorable progression of the disease was more frequent in patients with AIT (39/40 patients, 97.5%) than in other therapies (15/27 patients, 55.6%) (p<0.001). Patients treated with AIT showed a significant decrease in IL-2R levels (p<0.001), while patients with conventional treatment showed no significant changes. Besides, patients with favorable progression showed a significant decrease in IL-2R levels, while patients with unfavorable progression shoved no significant changes (Table 2).
| N | Pre-treatment | Post-treatment | Significant | |
|---|---|---|---|---|
| All patients | 35 | 560.9 ± 204.6 | 399.1 ± 155.8 | 0.004 |
| Patients with AIT | 20 | 597.2 ± 161 | 362.4 ± 130.8 | <0.001 |
| Patients without AIT | 15 | 512.4 ± 249.1 | 448.1 ± 176.8 | 0.508 |
| Patients with unfavourable progression | 9 | 454.6 ± 232.3 | 425.8 ± 186.1 | 0.86 |
| Patients with favourable progression | 36 | 587.4 ± 192.5 | 392.5 ± 150.5 | 0.001 |
Table 2: Statistics of related samples, in patients with Pre and Post-treatment sIL-2R levels.
The measurement of IL-6 detected changes neither in AIT versus conventional treatment patients nor in favorable versus unfavorable disease progression (Table 3).
| N | Pre-treatment | Post-treatment | Significant | |
|---|---|---|---|---|
| All patients | 35 | 2.8 ± 1.5 | 2.9 ± 1.5 | 0.861 |
| Patients with AIT | 20 | 2.9 ± 1.4 | 2.9 ± 1.4 | 0.891 |
| Patients without AIT | 15 | 2.7 ± 1.6 | 2.9 ± 1.4 | 0.703 |
| Patients with unfavourable progression | 9 | 2.6 ± 1.5 | 3.1 ± 1.6 | 0.54 |
| Patients with favourable progression | 36 | 2.9 ± 1.5 | 3.1 ± 1.6 | 0.705 |
Table 3: Statistics of related samples, in patients with Pre and Post-treatment IL-6 levels.
Discussion
Eosinophilic esophagitis is a recognized allergic disease [4]. Research in recent years has contributed to a better understanding of EoE assessment of disease activity, but it is necessary to advance in the evaluation of minimally invasive diagnostic tools, and new therapeutic approaches [4,9]. Currently, the only way to diagnose EoE is with an endoscopy and biopsy of the esophagus, although the utility of a noninvasive serum biomarker paned for diagnosis and monitoring of eosinophilic esophagitis have been considered [11-14].
Although eosinophilic esophagitis is considered a hypersensitivity reaction, in vivo allergic tests (skin prick tests) do not give ought diagnosis and prognostic information [4].
Eliminating major food allergens from the diet before any food allergy testing is an accepted treatment of EoE. The foods excluded usually include dairy, egg, wheat, soy, peanut, tree nuts, and fish/shellfish. These diets help treat EoE, although they can be very difficult to follow. Foods are typically added back one at a time with follow-up endoscopies to make sure that EoE remains in control but relapse is very frequent [1,2,4].
No medications are currently approved by the US Food and Drug Administration (FDA) to treat EoE. However, medications have been shown to reduce the number of eosinophils in the esophagus and improve symptoms. Corticosteroids, which control inflammation, are the most helpful medications for treating EoE. Swallowing small doses of corticosteroids is the most common treatment. At first, higher doses may be needed to control the inflammation but the higher doses are linked with a greater risk of side effects. Proton Pump Inhibitors (PPIs), which control the amount of acid produced, have also been used to help diagnose and treat EoE. Some patients respond well to PPIs and have a large decrease in the number of eosinophils and inflammation when a follow-up endoscopy and biopsy are done. However, PPIs can also improve EoE symptoms without making the inflammation any better [4,9].
We have successfully treated patients who suffered from eosinophilic esophagitis whit Allergen Specific Immunotherapy (AIT). The monitoring of this efficiency has been carried out with clinical parameters and serial endoscopies, but it would be useful to find a serological marker with a good response to the AIT that would obviate them.
sIL-2R has proven a useful monitor for any active eosinophilic inflammatory diseases (autoimmune, neoplasic, and infectious), but it has not been measured in Eosinophilic Esophagitis (EoE). IL-6 has a great inflammatory activity more associated with rheumatoid arthritis but could measure the lymphocytic and fibrotic response that occurs in the chronification of this pathology.
In all ways of treatments should be necessary to monitor their efficacy. The most common methods in EoE are based on clinical subjective scores and endoscopy with biopsy. We proposed a simple analytical method measurement of sIL-2R. The receptor of the cytokine interleukin-2 plays a crucial role in the regulation of the immune response. The binding of IL-2 to its receptor on the surface of T-lymphocytes triggers a series of intracellular signaling events that result in the activation and proliferation of resting T-cells and the generation of helper and suppressor T-cells (in the same way of AIT). Most resting T-cells, B cells, Large Granular Lymphocytes (LGLs), and monocytes do not express significant numbers of this receptor on their surfaces. Upon activation, receptor molecules are expressed on the surface of the cells, and a soluble form (sIL-2R) is released, which is about 10 kDa smaller than the membrane-bound protein. It has been found that sIL-2R is present at low levels in serum of healthy individuals and significantly elevated levels in a broad range of disorders such as cancer diseases, autoimmune diseases, organ allograft rejection, and different infections, but their levels had not been studied in patients suffered from eosinophilic esophagitis.
In our study, we found that the favorable progression of eosinophilic esophagitis was more frequent in patients treated with AIT (39/40 patients, 97.5%) than in treatment with other therapies (15/27 patients, 55.6%) (p<0.001). Thus, patients treated with AIT showed a significant decrease in sIL-2R levels (p<0.001), while patients with conventional treatment did not show significant changes. Also, patients with favorable progression showed a significant decrease in sIL-2R levels, while patients with unfavorable progression did not show significant changes [11,12,14].
The measurement of IL-6 did not detect changes in AIT compared to patients with conventional treatment, nor in the progression of the favorable versus the unfavorable disease [11,14].
Several unmet needs are to be solved urgently in EoE, as finding non-invasive disease-monitoring methods and biomarkers for routine practice, the development of new therapies, novel food allergy testing to detect triggering foods, drug, and doses required for initial therapy and safety issues with long-term maintenance therapy, amongst others. Besides, multidisciplinary management units of EoE, involving gastroenterologists, pediatricians, allergists, pathologists, dietitians, and clinical analysis specialists are needed.
Conclusion
Measurement of sIL-2R can be useful in monitoring the efficacy of specific immunotherapy in EoE as can be used also as a marker of the activity of the disease, but as a diagnostic test, it is not valid, while IL-6 is not valid neither for monitoring nor as a diagnostic test.
Declarations
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Armentia, A., et al. "Value of microarray allergen assay in the management of eosinophilic oesophagitis."Allergologia Et Immunopathologia,Vol. 43, No. 1, 2015, pp. 73-80.
- Armentia, Alicia, et al. "Germination of pollen grains in the oesophagus of individuals with eosinophilic oesophagitis."Clinical and Experimental Allergy,Vol. 49, No. 4, 2019, pp. 471-73.
- Sastre, B., et al. "Eosinophils: Old players in a new game."Journal of Investigational Allergology and Clinical Immunology,Vol. 28, No. 5, 2018, pp. 289-304.
- Mogensen, Ida, et al. "Fixed airflow obstruction relates to eosinophil activation in asthmatics."Clinical and Experimental Allergy,Vol. 49, No. 2, 2019, pp. 155-62.
- Roquet, A., et al. "Eosinophil activity markers in peripheral blood have high predictive value for bronchial hyper-reactivity in patients with."Allergy,Vol. 51, No. 7, 1996, pp. 482-88.
- Gomez Torrijos, Elisa, et al. "Eosinophilic esophagitis: Review and update."Frontiers in Medicine,Vol. 5, 2018, p. 247.
- Tanaka, Toshio, Masashi Narazaki, and Tadamitsu Kishimoto. "IL-6 in inflammation, immunity, and disease."Cold Spring Harbor Perspectives in Biology,Vol. 6, No. 10, 2014, p. a016295.
- Zhang, Zinan, et al. "Human interleukin-2 receptor ß mutations associated with defects in immunity and peripheral tolerance."The Journal of Experimental Medicine,Vol. 216, No. 6, 2019, pp. 1311-27.
- Ram, Gita, et al. "Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis."Annals of Allergy, Asthma and Immunology,Vol. 115, No. 3, 2015, pp. 224-28.
- National Committee for Clinical Laboratory Standards. "Procedures for the collection of diagnostic blood specimens by venipuncture; approved standard." 4th ed. NCCLS Document H3-A4, Wayne, PA: NCCLS, 1998.
- Arias, Ángel, et al. "Toll-like receptors-mediated pathways activate inflammatory responses in the esophageal mucosa of adult eosinophilic esophagitis."Clinical and Translational Gastroenterology,Vol. 9, No. 4, 2018, p. 147.
- Dellon, Evan S., et al. "Utility of a non-invasive serum biomarker panel for diagnosis and monitoring of eosinophilic esophagitis: A prospective study."The American Journal of Gastroenterology,Vol. 110, No. 6, 2015, pp. 821-27.
- Hatayama, Takahiro, and Noriyasu Hirasawa. "TSLP-basophil axis."Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica,Vol. 143, No. 2, 2014, pp. 103-04.
- Blanchard, Carine, et al. "A striking local esophageal cytokine expression profile in eosinophilic esophagitis."Journal of Allergy and Clinical Immunology,Vol. 127, No. 1, 2011, pp. 208-17.