Research - International Journal of Medical Research & Health Sciences ( 2022) Volume 11, Issue 10
A Study on Risk Factors, Immediate Outcome and a Short Term Follow Up Study of Neonates With Neonatal Thrombocytopenia
N. Ragavendran, N. Kumar* and V. ThirunavukkarasuN. Kumar, Department of Pediatrics, Government Medical College Dindigul, Tamil Nadu, India, Email: drnkumarmdp@gmail.com
Received: 05-Oct-2022, Manuscript No. ijmrhs-22-76578; Editor assigned: 10-Oct-2022, Pre QC No. ijmrhs-22-76578 (PQ); Reviewed: 11-Oct-2022, QC No. ijmrhs-22-76578 (Q); Revised: 13-Oct-2022, Manuscript No. ijmrhs-22-76578 (R); Published: 30-Oct-2022
Abstract
Background: Neonatal Thrombocytopenia, a platelet count <1.5 lakhs/µL is one of the most common hematological problems in a Neonatal Intensive Care Unit (NICU), with 18%-35% prevalence. More common among ELBW or Preterm babies (GA<or=32-36 weeks) or Sick neonates. Whereas, only 2% are thrombocytopenic at birth in term NB, and Severe Thrombocytopenia (platelet count <50,000 /µL) occurs in less than 3/1000 term infants. Objectives: To determine etiology, various comorbid conditions, onset, clinical features, immediate outcome, and a short term follow up of the Neonates. Methods: It was a Prospective Observational Study done with 140 neonates from May 2021 to Jun 2022, admitted to the NICU of a Tertiary Level Medical College Hospital in South Tamil Nadu, India. Neonates showing bleeding/having platelet count (<1.5 Lakhs/µL) were selected. Initial platelet count done on admission and counts repeated 12 hours after any therapeutic intervention. Treatment was given as per NICU protocols. Results: Mucosal bleed was the most common presentation. Severe Thrombocytopenia (<50,000/µL) was present in 08.5%, Moderate (<100,000/µL and ³ 50,000/µL) in 17%.45. 33% were preterm, 23% IUGR, 29% were LBW, 51.3% had Septicemia, 29.4% Birth asphyxia, 19% MAS, 11.1% DIC and 14% had NEC. 33.3% presented early (<72hrs) and 60% presented late (>72hrs). 21.5% of EOS and 55% of LOS developed severe thrombocytopenia. 22.33% were given Platelet Concentrate. Mortality was 37% in the Severe and 3.9% in the Moderate thrombocytopenia group. A significant association was observed with Maternal PIH, LOS, NEC, and Sepsis with DIC. Prematurity, IUGR, and Birth asphyxia were commonly associated with morbidities. Factors leading to Birth asphyxia and Sepsis directly influence platelet counts. Conclusion: Severe thrombocytopenia, in sick neonates in NICU, is a poor prognostic indicator.
Keywords
Neonate, Preterm, Thrombocytopenia
Introduction
Thrombocytopenia (platelet count <150,000/µL) is the second most common hematological problem in Neonatal Intensive Care Units, with an 18%-35% prevalence rate and first being phlebotomy-induced anemia [1]. It is more common among Extremely Low Birth Weight neonates (<50,000/µL) occurs in <3/1000 term infants [2, 3].
Aims and Objectives
Aimed to find out current data on the outcome (mortality and morbidity) and a short-term follow-up study of thrombocytopenic neonates (<1 50,000/µ L) in our hospital. Secondary objectives were:
• To study maternal and fetal risk factors and the clinical course of neonatal thrombocytopenia during the hospital stay.
• To evaluate the role of neonatal thrombocytopenia as a prognostic indicator in NICU graduates.
• To objectively judge the efficacy of treatment protocol practiced in our NICUs to manage thrombocytopenic neonates.
Background and Justification
The influence of thrombocytopenia on the outcome of the neonate is a subject that has not been studied in detail in the past, and neither have articles assessed the value of neonatal thrombocytopenia as a prognostic indicator in sick neonates. After a detailed search of the indexed medical literature, it was found that there have been only a few articles on this topic from India [4, 5]. One article is a study of the association between maternal PIH and neonatal thrombocytopenia while the others are case reports and case series reports [6]. The paucity of studies from India and the increasing prevalence of this condition in our NICU instigated us to do this study.
Platelets are small anucleate fragments that are formed from the cytoplasm of Megakaryocytes and have a characteristic discoid shape [7]. Platelet production begins in the yolk sac and, shifts to the fetal liver and then to the marrow during gestation [8]. Megakaryocytic progenitor cells proliferate, under the influence of Thrombopoietin and growth factors with Megakaryocyte colony-stimulating activity, into Megakaryoblasts which further differentiate into Megakaryocytes. All the major Megakaryoctyes progenitor and precursor cells have been identified in the fetus and the newborn [9, 10]. Tpo initiates the maturation program that amplifies the Megakaryocyte's DNA and leads to the synthesis of platelet-specific proteins [11]. The magnitude of the proliferative and maturation responses to cytokines is related to developmental age [12]. Reticulated platelet counts are increased in premature neonates less than 30 weeks of gestation. But generally, neonatal progenitors have a higher clonogenic potential than adult megakaryocyte progenitors [13]. Platelets appear in fetal circulation from the 5 th week of gestation and by the 2nd trimester, the adult platelet count of 150/µL-450,000/µL reached [14]. Similar to adults the principal hematopoietic growth factor controlling megakaryopoiesis is Tpo or c-mpl ligand. Megakaryocyte precursors and progenitor cells from term and preterm infants proliferate extensively in response to exogenous recombinant Tpo [12]. The premature neonate may be a platelet count below 150,000/µL but it cannot be considered to be normal [15]. The size of the platelet in the term and premature infant averages 7 fl-9 fl similar to the adult normal range [7]. Thrombocytopenic babies had decreased platelet survival time compared to that normal adult [16]. Neonatal Thrombocytopenia classified into mild (<150,000/µL and 100,000/µL), mod (<100,000/µL and 50,000/µL), severe (<50,000/µL) [1, 2]. Most of the studies show that among NICU patients, (75%-90%) of neonatal thrombocytopenia is either present at birth or develops within 72 hours. These neonates have a predictable chronological pattern of thrombocytopenia where rarely the platelet count falls below 100,000/µL reaching its nadir by 4 days to 5 days and recovering to more than 150,000/µL by the end of the 1st week [17]. Most of these patients are either preterm neonates or neonates with a history of placental insufficiency with fetal hypoxia. This type of thrombocytopenia is referred to as early neonatal thrombocytopenia. Neonatal thrombocytopenia is a risk factor for spontaneous Intracranial Hemorrhage (ICH), especially Intra-Ventricular Hemorrhage (IVH) [18-24]. There is a significant risk of ICH when the platelet count drops below 50,000/ µL15. Thrombocytopenic infants are also at greater risk for developing other abnormal bleeding such as gastrointestinal, pulmonary, and cutaneous bleeds. And all the thrombocytopenic neonates may have disease processes historically associated with increased platelet consumption (sepsis, asphyxia, maternal placental insufficiency, and ECMO use) [23]. It has been shown that the Thrombopoietin (TPO) levels in thrombocytopenic preterm infants were not significantly different from those of nonthrombocytopenic preterm neonates, unlike older children in whom usually thrombocytopenia is associated with elevated TPO levels. In Preterm NB, thrombocytopenia is associated with IUGR and a history of maternal PIH. The underlying cause of thrombocytopenia in preterm neonates with a history of placental insufficiency may be impaired platelet production due to insufficient Tpo secretion. The dose-dependent proliferation of neonatal megakaryocyte precursors to TPO has been proven both in vitro and in vivo. TPO levels did not correlate either with gestation age or with platelet counts [25]. A better inverse correlation was found with IUGR who had both decreased TPO levels and megakaryocyte mass. Hypoxia is not directly but indirectly influence the hematopoietic microenvironment and depresses the megakaryocyte clonogenic maturation [26-28]. These findings show that in case of prematurity and associated placental insufficiency there is impaired platelet production that is neither due to an immature TPO synthesis system with reduced baseline levels nor due to insensitivity to TPO, rather it is either due to an attenuated TPO response in the time of need or due to the deleterious effects of hypoxia and prematurity on the hematopoietic microenvironment [25,28]. These findings do not necessarily mean that impaired platelet production is the only cause and consumption is not, it just shows that a combination of factors predisposes sick neonates to thrombocytopenia and impaired platelet production is one of the underlying factors in the majority of the cases [25]. There are other conditions with impaired platelet production. Some of them are Congenital megakaryocytic thrombocytopenia, Thrombocytopenia Absent Radii (TAR) syndrome, Fanconi’s anemia, trisomies of chromosome 13, 18, or 21 and Turners syndrome, Bernard Soulier Syndrome, radioulnar synostosis, May Heggins anomaly, and Sebastian Syndrome, Congenital dysmegakaryopoietic thrombocytopenia (Paris Trousseau) syndrome. Current evidence shows that most of the platelet consumptive disorders in neonates are immunologically mediated, 15%-20% of all early thrombocytopenias are due to transplacental passage of maternal platelet allo and autoantibodies [29-33]. DIC is responsible for thrombocytopenia in some sick newborns (prenatal asphyxia/sepsis) but contrary to the popular belief that DIC is the most common cause of thrombocytopenia in the NICU, reports show that it occurs in only 10%-15% of neonatal thrombocytopenia [34-37]. There is also evidence of limited splenic sequestration of platelets occurring in sick newborns. Thus platelet consumption and sequestration together are likely to be a major mechanism of thrombocytopenia in well under 50% of neonatal thrombocytopenia cases [38]. In neonates, DIC usually complicates severe illness, such as bacterial/viral sepsis, HMD, MAS, or asphyxia 25. Thrombocytopenia is a consistent finding, prolonged PT, APTT-increased FDP, and increased D-dimers with decreased fibrinogen levels are other lab abnormalities [39]. In 40%-50% of cases of DIC, the platelet count falls below 50,000/µL. 80%-90% of neonates with gram-negative sepsis and fungemia had lower mean platelet counts compared to that neonates with gram-positive sepsis [40].
Thrombocytopenia can occur in neonates who have localized thrombi such as in the renal vein, or sagittal sinus, attached to an indwelling catheter/or an ECMO circuit [38]. This leads to Thrombocytopenia by increased consumption of platelets, either by the progressive aggregation to a developing Thrombus or platelet destruction due to interaction between the platelets and any pathological surface in the vascular system38. Thrombosis or platelet activation or immobilizations at sites of inflammation as in NEC, hemangiomas (Kasabach-Merritt syndrome), and indwelling umbilical catheter are other examples of neonatal thrombocytopenia principally due to platelet consumption [39-43]. Other genetic disorders cause consumptive platelet loss, like congenital TTP there is consumptive platelet loss. Pseudo vWD and type IIb vWD: (Platelet type vWD), both are similar disease entities where platelets aggregate in the presence of lower than normal concentration of ristocetin and decreased circulating vWF48. Most infants acquire thrombocytopenia due to multiple concurrent mechanisms, [44-46]. Congenital TORCH infections particularly CMV produce thrombocytopenia8. Though thrombocytopenia is common in women with HIV, thrombocytopenia is quite rare in HIV-infected neonates of these women8. Coxsackie B can cause fulminant hepatitis, thrombocytopenia may be evident at birth, and myocarditis and CNS involvement may or may not be associated49. Congenital echovirus, parvovirus B 19, mumps, EBV, and adenovirus infections have been reported to cause thrombocytopenia [47-50]. The mechanism in congenital viral infection is most probably a combined mechanism producing both decreased production and increased consumption 38. Vacuolization of megakaryocytes has been reported in these cases suggesting decreased megakaryopoiesis, splenomegaly, and increase RES Activity8. Increased platelet sequestration can lead to consumption. Endothelial damage can also contribute to increasing platelet removal2. Wiskott Aldrich syndrome and X- linked thrombocytopenias are associated with allelic mutations in the WASP gene at X p11.22-p11.23. Wiskott-Aldrich syndrome Thrombocytopenia is associated with bleeding, eczema, and immunodeficiency. It also has an X-linked inheritance 50. Numerous drugs have been reported to cause neonatal thrombocytopenia, but only some have been implicated beyond statistical probability [38]. It might involve an immune reaction in which an antibody against a hapten complex cross-reacts with platelet antigens quinine, quinidine, hydralazine, tolbutamide, and thiazide diuretics. Low-dose infusion of LMW heparin is practiced to provide potency for central venous catheters in sick neonates. This might lead to heparin-induced thrombocytopenia [51]. Heparin-induced thrombocytopenia is common in NICU, a report studied 34 such cases and 14 of them had heparin-associated anti-platelet antibodies. Just like most of the other drug-induced thrombocytopenias, it is associated with an immune reaction to a drug-hapten complex that produces thrombocytopenia. They are usually treated with either low-dose danaparoid sodium or lepirudin.Other drugs that have been rarely associated with neonatal thrombocytopenia are vitamin A, continuous glucagon infusion, maternal phenytoin, benzyl alcohol, and parenteral lipids [8]. Transient Thrombocytopenia occurs with indomethacin but usually is mild and resolves in 2 days-3 days [52].
Clinical Features and Risk Factors
Clinical features are petechiae, bruising, hematuria, and bleeding from the ET tube. Risk Factors observed in various studies were RDS, asphyxia, Sepsis, Hypoxia, presence of an umbilical catheter, phototherapy, respiratory assistance, hyperbilirubinemia, prematurity, MAS, NEC, maternal hypertension, Absent umbilical artery end diastolic flow (placental insufficiency) in the growth-restricted fetus, polycythemia, mechanical ventilation, heparininduced, indomethacin therapy, exchange transfusion, TPN. No relationship between the degrees of thrombocytopenia to clinical features was detected. Significantly more thrombocytopenic babies (22% vs 3%) had skin, renal, pulmonary, or CNS hemorrhage compared to nonthrombocytopenic neonates23. Modified template bleeding time was inversely related to platelet count. The hemorrhage score was greater in thrombocytopenic infants [53]. The incidence of IVH (78% vs. 48%) was more in thrombocytopenia cases. Thrombocytopenic neonates are at greater risk for bleeding compared to nonthrombocytopenic but equally sick neonates. There is less likelihood of ICH when the platelet count is maintained above 50,000/µL. It is likely to be pathological other than early neonatal thrombocytopenia when the platelet count falls below 50,000/µL in the first three days and doesn’t recover by 7days-10 days [54]. The most important cause of such thrombocytopenias are neonatal allo and autoimmune thrombocytopenia, especially the former, its incidence is 1 in 1500 pregnancies. In NAIT, in 75% of cases, the thrombocytopenia is severe and might last longer than 7 days-10 days [55-58]. They require active intervention. In NICU patients’ thrombocytopenia after 72 hours, almost always results from late-onset sepsis or NEC [59-61]. It also has a typical pattern, rapid onset, and progression, and the nadir is reached in 24 hours-48 hours. As sepsis or NEC is controlled platelet numbers slowly recover by 5 days-7 days [62-65]. Thrombocytopenia is usually severe with counts falling below 50,000/µL and requiring active intervention. Immune thrombocytopenias in the neonate can either be due to transplacental passage of maternal platelet alloantibodies (neonatal alloimmune thrombocytopenia) or due to the passage of maternal autoantibodies to platelets, in mothers with ITP, SLE, and antiphospholipid antibody syndrome [66-68]. The pediatric hematology committee of the American association of blood banks, recommends <50,000/µL and <100,000/µL as the trigger threshold in stable preterm and sick preterm respectively. The British counterpart, the committee for standards in neonatal transfusion task force recommends much lower counts of <30,000/µL to <20,000/µL respectively [69]. Thrombocytopenia is more of a disease marker of prognostic value rather than a pathologic process producing excessive morbidity and mortality.
Methodology
Study Design
Prospective observational study.
Source of Data
140 of 550 consecutive neonates admitted to the NICU of Tertiary Level Medical College Hospital South Tamil Nadu, between May 2021 and Jun 2022 were taken up for the study. All the neonates, except those who were lost for follow-up and those who expired, were followed up after their discharge for 3 months.
Exclusion Criteria
Those neonates, with major congenital malformations, Babies born outside our hospital and admitted to our NICU (extramural). H/o maternal medications like aspirin, warfarin, congenital cardiac malformations, and surgical conditions in neonates.
Method of Collection of Data
At admission, the parents and/or the guardian were informed about the study and oral informed consent was obtained. A detailed history inclusive of maternal history and obstetric history with a focus on history suggestive of bleeding and its type in the newborn or the mother was obtained as per the proforma. Information regarding several conditions that have been implicated by past studies to be associated with neonatal thrombocytopenia was prospectively recorded. A history of PIH, gestational diabetes mellitus, premature rupture of membrane, and Rh isoimmunization in the mother was asked for. These diagnoses were made as per the standard diagnostic criteria laid down. History of consumption of drugs by the mother that can predispose her to neonatal thrombocytopenia was also documented. The gestational age of all neonates was determined based on New Ballard’s scoring system. Growth assessment at birth or admission to detect intrauterine growth restriction was based on Fenton Intrauterine growth charts. Every neonate had a detailed physical examination as in the proforma with a focus on purpuric/petechial rashes, mucosal bleeding, etc. Other common sites of bleeding were also looked for. All the neonates underwent necessary blood investigations, viz. Complete blood count, Peripheral smear study, Blood culture, Septic workup (Absolute neutrophil count, Total WBC count, Micro ESR, C reactive protein). Blood was collected in sterile EDTA bulbs by venepuncture after taking all aseptic precautions and swiftly transferred to Central Laboratory the time lag between collection and estimation was usually 10 to 15 minutes. CBC was obtained from an automated hematology analyzer. Peripheral smear study and blood cultures were done using standard laboratory methodology. A Septic workup inclusive of the absolute neutrophil count, Total WBC count, Micro ESR, and C reactive protein was done on all patients. If any two of the above-mentioned were positive then the neonate was labeled as having suspected septicemia. Quantitative determination of CRP was done by latex turbidimetry using Spinreact Crp-Turbilatex. A value of more than 0.6 mg/l was considered abnormal. Based on their platelet counts at admission, the neonates were grouped as Group I/Nonthrombocytopenics>150,000/µL, IIa/Mild to moderate thrombocytopenia between <150,000/µL and 50,000/µL, IIb/Severe thrombocytopenia<50,000/µL. Most of the cases in group lIb investigations such as PT and aPTT were done. 1.8 ml of venous blood was collected in a bottle containing 0.2 ml of 3.8% sodium citrate so that the ratio of blood and citrate is 9:1. In the present study PT, and aPTT were obtained by an automated coagulation analyzer. (Normal PT:14 sec-22 sec, Normal aPTT: 30 sec-55 sec). Platelet counts were repeated 24 hours after medical interventions in all cases. Other investigations such as chest X-ray, neurosonogram, and Computed Tomography of the brain were performed whenever the need was raised. Due to a lack of laboratory facilities, tests for platelet alloimmunization were not conducted on all suspected cases as per recommendations. All diagnoses were based on standard diagnostic criteria laid down in indexed medical literature. All the neonates were managed according to standard NICU protocol as per recent recommendations in the medical literature. On the day of discharge, all the neonates underwent a detailed clinical examination. Immediate outcome defined as Satisfactory: (If the patient fulfills all the criteria: All acute problems should have become passive, Baby should be accepting breastfeeding or paladar feeds, Adequate weight gain for 3 consecutive days, At discharge baby weight, should be more than or equal to 1.5 kg, There is no associated morbidity such as hypoxic-ischemic encephalopathy, persistent seizures, intracranial bleed, etc.), Not satisfactory(If the patient does not fulfill even one of the above-mentioned criteria), Expired. All neonates were followed up at least once for 3 months. During each visit, a detailed physical examination was done. Weight and head circumference were measured and plotted on the growth chart and development chart. The growth parameter at a point of time lying below the 3 rd percentile was considered to be abnormal.
Neurodevelopment was assessed using the Denver II scale. It is not designed to be an IQ test nor can it generate diagnostic labels such as learning disability, language disorder, etc. Rather the test is designed to compare a given child’s performance on a variety of tasks to the performance of others of the same age. Denver II consists of 125 tasks/ items which are arranged on the test forms in 4 sectors:
• Personal-social
• Fine motor- Adaptive
• Language
• Gross motor
The Denver II is interpreted as follows
Normal: No delays and a maximum of one caution in the various test items.
Suspect Two or more cautions and/or 1 or more delays. Based on their physical growth and neurodevelopment in the 3rd month, the neonates were classified as having a good (g), fair (f), poor (p), and expired (e) outcome. Infants were classified as having a poor outcome if their neurodevelopment outcome was suspect, as per the Denver II development scale, on two different occasions, irrespective of their physical growth. Any mortality was recorded both at discharge and at follow-up (Table 1).
| Outcome | Denver II | Weight and/or head circumference |
|---|---|---|
| Good(g) | Normal(N) | = or ≥ 3rd centile |
| Fair(f) | Normal | < 3rd centile |
| Poor(p) | Suspect(S)/expired | <or> or = 3rd centile (irrespective of their growth) |
Statistical Analysis
Descriptive data are presented as numbers or percentages. Comparison of the groups for categorical variables was done by Chi-square test. Continuous variables were analyzed using unpaired two-tailed student t-tests or by one-way analysis of variance (ANOVA). A ‘P’ value below 0.05 was considered significant. The dependent variable was the outcome which was classified into good (0), fair, and expired or poor neurodevelopment irrespective of the physical growth.
Results
A total of 550 consecutive neonatal admissions were included in our study as per the inclusion and exclusion criteria laid down. The subjects were divided into three groups based on their platelet counts, as has already been mentioned.
Group I: No Thrombocytopenia-410(74.54%),
Group IIa: Mild to moderate Thrombocytopenia 93(17.01%),
Group IIb: Severe Thrombocytopenia- 47(08.5%).The mean platelet count for all the groups was 1.603 lakhs/µL with a standard deviation of ± 0.71.
Etiologic Diagnosis
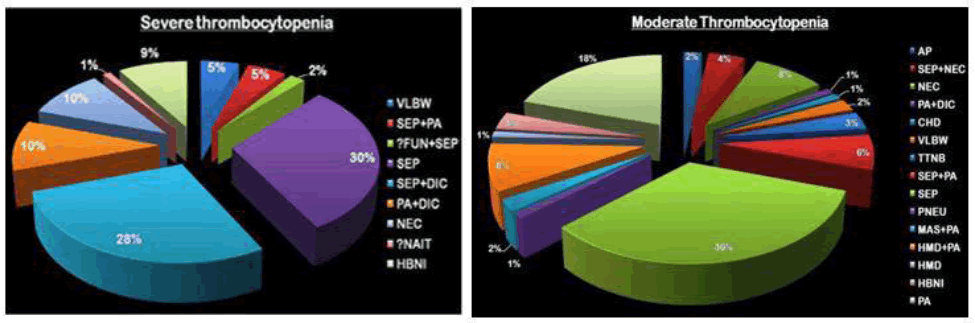
The most common diagnosis in the mild to moderate group was septicemia, while in the severely thrombocytopenic group it was Septicemia with Disseminated intravascular coagulation (Figure 1).
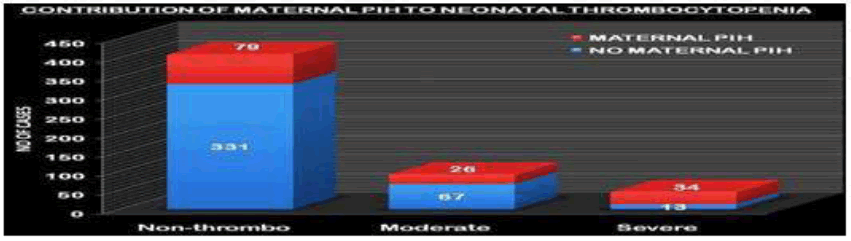
Maternal PIH
It was significantly associated with thrombocytopenia. In both groups, IIa and lIb the prevalence of maternal PIH was high (27.95% and 93.61%) respectively. The p-value obtained by the Chi-square method was <0.001 and it was highly significant. The odds ratio of thrombocytopenia and Maternal PIH was also calculated and it was 4.8 with a confidence interval of 2.4-9.3 (Table 2 and Figure 2).
| Group I % | Group Iia % | Group Iib % | ||||
|---|---|---|---|---|---|---|
| PIH+ | 79% | 19.44% | 26% | 27.95% | 34% | 72.34% |
| PIH- | 331% | 80.55% | 67% | 72.04% | 13% | 27.65% |
Other maternal factors such as gestational diabetes mellitus, Rh incompatibility, premature rupture of membrane, antepartum hemorrhage, place, and mode of delivery were not significantly associated with thrombocytopenia.
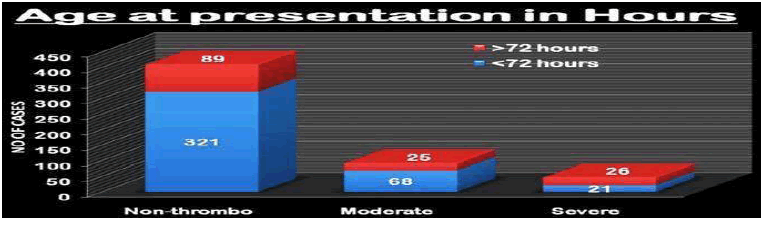
Age at Presentation
Severely thrombocytopenic neonates tend to present later than 72 hours compared with cases with mild to moderate thrombocytopenia and no thrombocytopenia. The ‘p-value for the association by Chi-square test was 0.012 and it was significant (Table 3 and Figure 3).
| Group I | % | Group IIa | % | Group IIb | % | |
|---|---|---|---|---|---|---|
| <72 hrs | 321 | 78.29% | 68 | 73.11% | 21 | 44.68% |
| >72 hrs | 89 | 21.7% | 25 | 26.88% | 26 | 55.31% |
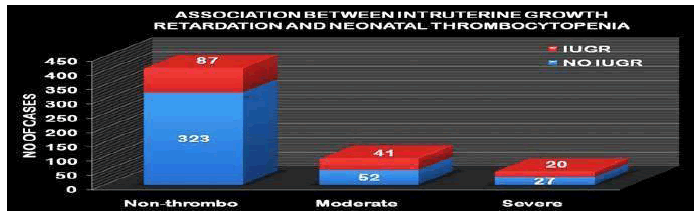
Intrauterine Growth Retardation
IUGR as assessed by the Fenton Intra uterine growth charts was significantly associated with thrombocytopenia (Table 4 and Figure 4).
| Group I | % | Group IIa | % | Group IIb | % | |
|---|---|---|---|---|---|---|
| NO IUGR | 323 | 78.78% | 52 | 55.91% | 27 | 57.44% |
| IUGR | 87 | 21.21% | 41 | 44.08% | 20 | 42.55% |
Septicemia
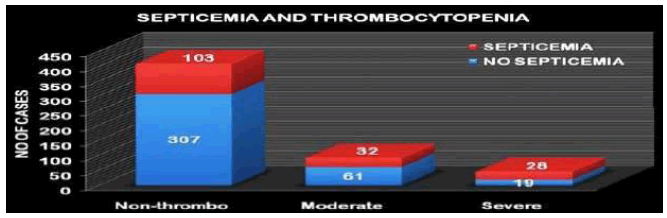
Septicemia was significantly associated with thrombocytopenia. While the prevalence of septicemia was 60% in the severely thrombocytopenic group, it was 34.40% and 25.12% in the mild to moderate and no thrombocytopenia groups respectively. Platelet counts in septicemia and nonsepticemic neonates were also compared, by student t-test, and the association was highly significant (Table 5 and Figure 5).
| Septicemia | Group I | % | Group IIa | % | Group IIb | % |
|---|---|---|---|---|---|---|
| No | 307 | 74.87% | 61 | 65.59% | 19 | 40.42% |
| Yes | 103 | 25.12% | 32 | 34.40% | 28 | 59.57% |
Assisted Ventilation
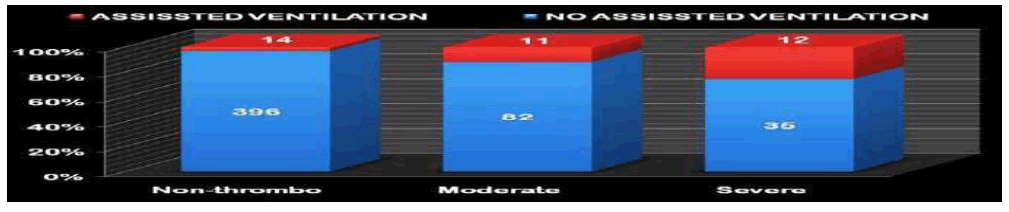
There were a significantly higher proportion of severely thrombocytopenic neonates on assisted ventilation. Other factors such as hypoxia, APGAR scores, hyperbilirubinemia, Meconium aspiration syndrome, and respiratory distress syndrome were not significantly associated with thrombocytopenia (Figure 6).
Platelet Count and Bleeding
To objectively assess the statistical relationship between platelet count and bleeding in neonates, the student T-test was used. The p-value was 0.001 which is significant (Table 6).
|
|
Mean platelet count (*10*/µL) | Number of neonates | |
|---|---|---|---|
| No bleed | 1.70 | 436 | T=3.72 |
| Bleed | 1.16 | 114 | P=0.001 |
DIC
Diagnosed by both an increase in PT, APTT was significantly associated with severe thrombocytopenia. While 25% of the severely thrombocytopenic cases had evidence of DIC only 1.9% had such laboratory findings in the mild to moderate thrombocytopenia group. The causes of DIC were either Perinatal asphyxia or Septicemia, accounting for 27.5% and 42.5% of the cases (i.e. cases of DIC) respectively.
NEC
NEC was significantly associated with thrombocytopenia with 11.8% and 17% of babies from Group IIa (moderate) and Group IIb (severe) respectively, being diagnosed with NEC. While there were 5.2% cases of NEC in Group I (non-thrombocytopenic. The p-value was <0.001 which is significant (Table 7).
| Group I (Non- throm) |
% with group |
Group IIa (Moderate) |
% within group |
Group IIb (Severe) |
% within group |
|
|---|---|---|---|---|---|---|
| NEC | 21 | 5.2% | 11 | 11.8% | 8 | 17.0% |
Intra Cranial Bleeding
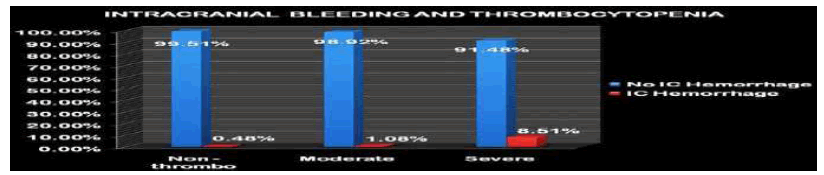
Instances of intra-cranial hemorrhage were higher in group IIb, the severely thrombocytopenic group, (8.51%) than in the other two groups. The statistical association between ICH and severe thrombocytopenia (ICH in group IIb Vs ICH in the other two groups) was detected and the association was significant (p<0.001) (Table 8 and Figure 7).
| Group I (Non- thrombo) | % with group | Group IIa (moderate) | % within group | Group IIb (severe) | within group % | |
|---|---|---|---|---|---|---|
| ICH | 2 | 0.48 | 1 | 1.075 | 4 | 8.51 |
| No ICH | 408 | 99.51 | 92 | 98.92 | 43 | 91.48 |
Signs and Symptomatology other than Bleeding
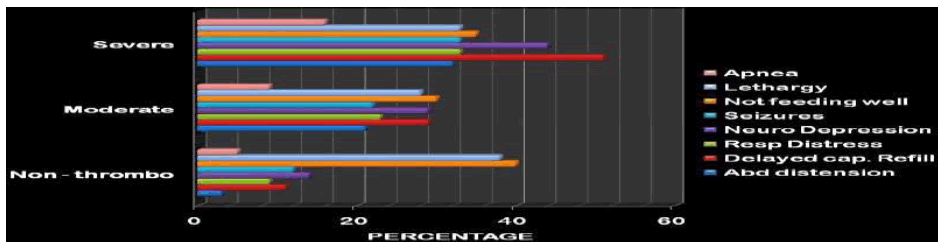
The most common symptom was not feeding well in all three groups. Evidence of delayed capillary refill, diagnosed as a shock, was significantly associated with severe thrombocytopenia. The prevalence of a capillary refill of more than 3 seconds was 50% in the severely thrombocytopenic group. The most common sign in the mild to moderate thrombocytopenic and no thrombocytopenia groups were signs of neurological depression. Evidence of GI bleed was also more prevalent in Group IIb but yet its association with the severe thrombocytopenia group was not statistically significant (Figure 8).
Course in the Hospital
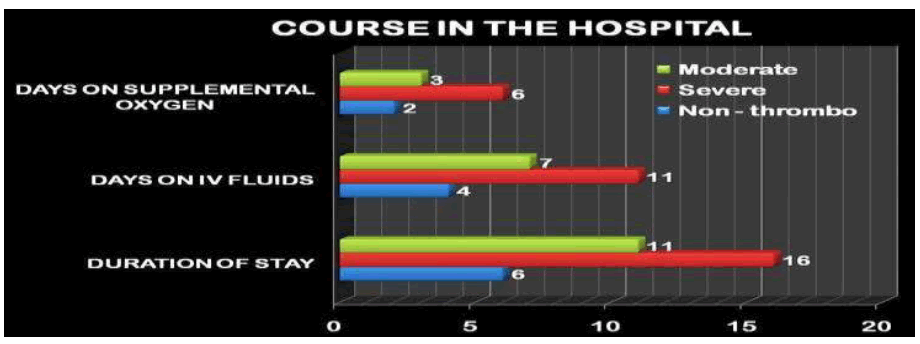
The number of days on Intra venous fluids, number of days on supplemental oxygen support, and duration of stay were evaluated. It was found that all three variables were significantly associated with thrombocytopenia and 87.5% of Group IIb (severe) babies and 82% of Group IIa (moderate) had to stay longer than a week while only 50% of the neonate in the Group I (not Thrombocytopenic) had to do so (Figure 9).
Mortalit
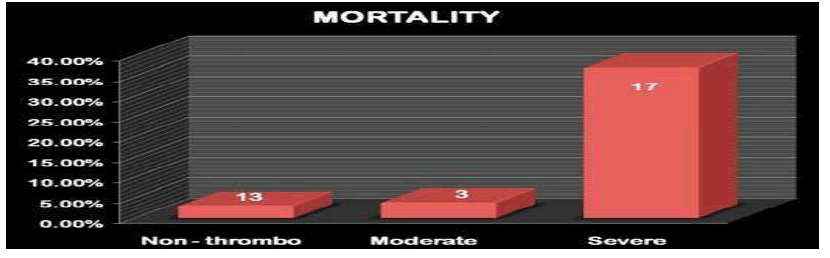
The mortality was significantly high in the severely thrombocytopenic group (37.12%) compared to the other two groups 3.15% and 3.92% respectively. The distribution of mortality based on the etiology in all the 3 groups is depicted in the table below and septicemia was the most common cause of death on the whole and in the severely thrombocytopenic group, accounting for 39.93% of the mortality (Figure 10 and Table 9).
| Mortality | Septicemia | MAS | RDS | Per. Asp | Con. An | Prematurity | Total |
|---|---|---|---|---|---|---|---|
| Group I | 4 | 3 | 1 | 0 | 0 | 5 | 13 |
| Group IIa | 1 | 1 | 0 | 0 | 0 | 1 | 3 |
| Group IIb | 8 | 2 | 2 | 2 | 1 | 2 | 17 |
| Mortality | 39.3 | 18.1 | 9.09 | 6.06 | 3.03 | 21.21 | 33 |
Immediate Outcome
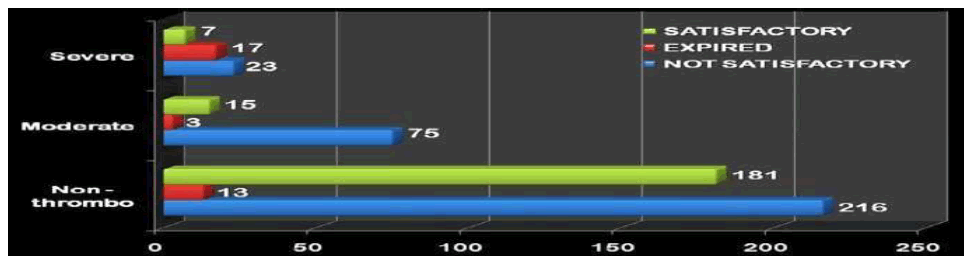
While the proportion of mortality was high in the severely thrombocytopenic group. The proportion of babies with a not satisfactory immediate outcome was higher in the mild to moderate thrombocytopenic group and no thrombocytopenia group (Figure 11 and Table 10).
| Immediate outcome | Group I (Non-thrombo) | % with group | Group IIa (moderate) | % within group | Group IIb (severe) | % within group |
|---|---|---|---|---|---|---|
| Expired | 13 | 3.15% | 3 | 3.92% | 17 | 37.12% |
| No satisfactory | 216 | 52.77% | 75 | 80.64% | 23 | 48.93% |
| Satisfactory | 181 | 44.14% | 15 | 16.12% | 7 | 14.89% |
Out of the 140 cases, there were 20 mortalities, and 18 of them were lost for follow-up. Those 18 cases that were lost for follow-up were excluded while assessing the outcome in the different groups. The outcome was classified as good, fair, poor, and expired, based on physical growth and neurodevelopment (Table 11)
| Group | Mortality | Lost for followup | Regularly follow up |
|---|---|---|---|
| IIa moderate | 3(3.92%) | 8 | 82 |
| IIb severe | 17(37%) | 10 | 20 |
Neurodevelopment
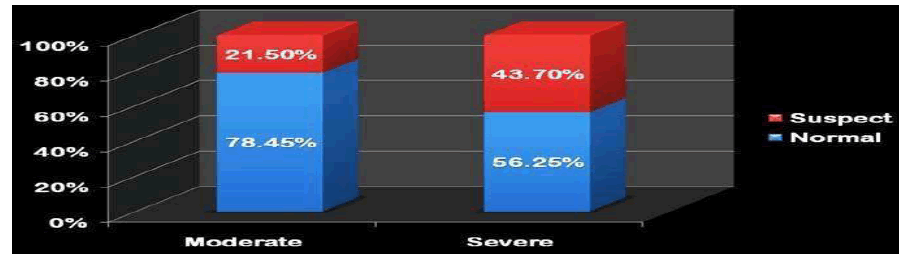
Among the cases followed up, the proportion of cases with the poor neurodevelopmental outcome (i.e. suspect outcome in Denver II) was higher in group lIb, i.e. severely thrombocytopenic group, (43.70%) than the other group (21.50 %) (Figure 12).
Percentage Increment in Platelet Count
The % increment in platelet count after 24 hours of blood transfusion, platelet transfusion, and exchange transfusion is illustrated graphically below. The comparison was done using student paired t-test and there was no significant difference (Table 12).
Discussion
e prevalence of thrombocytopenia in our study was 25.45%, whereas Castle et al16 study is 22%, and Hale Oren et al studies it is 5.4%. Beiner at al estimated the prevalence of thrombocytopenia as 31% only among preterm neonates [20,23]. The higher prevalence in our study is probably due to the higher proportion of septicemic neonates in our NICU admissions, 31.3%, while it was lower in the other studies, e.g. in the study conducted by Castle et al the prevalence of septicemia was just 7.5% [16]. The proportion of severe thrombocytopenia among neonatal thrombocytopenias, 33.57% in our study, is also on the higher side. This is once again probably a reflection of a higher contribution of septicemia to neonatal thrombocytopenia in our NICU than other etiologies. Septicemia is reported to result in severe thrombocytopenia rather than its milder form in various studies40. The mean platelet count among our NICU admissions was 1.603 × 105/µl. This is once again on the lower side compared to other studies. This might just be a reflection of the higher prevalence of severe thrombocytopenia in our NICU. The etiological profile on the whole was similar to other NICU studies from India, with septicemia and perinatal asphyxia accounting for the majority of the admissions. Septicemia accounted for most of the cases in both the severe and mild to moderate thrombocytopenia groups. Perinatal asphyxia accounted for most of the admissions in the no thrombocytopenia group. Septicemia with DIC accounted for 28% of the cases of severe thrombocytopenia. Septicemia leads to thrombocytopenia due to both decreased production and increased consumption of platelets and hence results usually in severe thrombocytopenia [25]. Probably, due to the higher incidence of septicemia in our admissions, the neonates with prematurity, IUGR, and perinatal asphyxia had also been exposed to infections more frequently than neonates with the same problems in western countries. Hence in neonates with an already compromised hematological environment exposure to infection probably leads to a precipitous fall in platelet count resulting in severe thrombocytopenia rather than its milder variety. Maternal PIH was significantly associated with neonatal thrombocytopenia (p<0.00l) and the odds ratio of the association was calculated to be 4.8 (with a C.I of 2.4-9.3). This finding is in agreement with studies conducted by Burrows et a155. 68.1% VS Our study72.17%. But maternal PIH is associated with mild to moderate thrombocytopenia rather than severe thrombocytopenia in other studies while in our study it was associated with severe thrombocytopenia. This could once again be explained by the frequent exposure of these neonates to infection, due to the relatively high prevalence of septicemia in our study that leads to a precipitous fall in platelet count. Other maternal factors such as PROM were not associated with neonatal thrombocytopenia in our study, unlike certain studies where an association has been documented20. It was shown that 55.31% of the severely thrombocytopenic neonates presented after 72 hours of life while only 25% and 21.7 % of neonates did so in the other two groups. This finding once again reiterates the well-documented association that the majority of the severely thrombocytopenic neonates present after 72 hours and the common etiology, in these neonates, are acquired ones such as septicemia and NEC2, 25. IUGR was not associated with thrombocytopenia, while in most of the western studies a strong association between the both has been reported21-23. Intrauterine growth Charts customized to Indian neonates might detect a more accurate prevalence of IUGR and then probably there could be an association between thrombocytopenia and IUGR [70-75]. Prematurity is known to be associated with lower platelet counts [20-23]. But in our study gestational age was not associated with low platelet counts. This is probably because most of the term neonates, in our study, were sick and septicemic about their premature counterparts, since many normal preterm neonates were admitted for preterm care, and their respective platelet counts may not reflect upon that of the normal term neonates. In western studies, only 10% of septicemia with severe thrombocytopenia had evidence of DIC whereas in our study it was 38.5% [2]. DIC is known to be initiated by bacterial toxins, such as exotoxins and lipopolysaccharide, producing endothelial dysfunction [76]. Quantitative and qualitative differences exist between the toxins secreted by various bacteriae. There was no association between gram-negative septicemia and thrombocytopenia in our study. While such an association has been shown to occur in certain studies, other studies give evidence to the contrary [40,41 ]. It might be because of interspecies variation in the pathological mechanism of septicemia among various gram-negative bacteriae [77]. Perinatal asphyxia is reported, widely, to be associated with neonatal thrombocytopenia. Perinatal asphyxia was diagnosed based on the following conditions in our study; an arterial pH of <7. Neonatal neurological manifestation suggestive of HIE, and evidence of multiorgan dysfunction. In our study, there was no significant association between perinatal asphyxia and thrombocytopenia. The reasons might be many, first of all, various studies adopted different definitions for perinatal asphyxia [78]. When there is no uniformity in the definition of perinatal asphyxia the correlation between it and thrombocytopenia cannot be compared across various studies. NEC, as diagnosed by Bell’s criteria, was significantly associated with thrombocytopenia (p<0.001). All the neonates in our study with radiological evidence of NEC had neonatal thrombocytopenia. This finding is in agreement with the well-known fact that thrombocytopenia is one of the major lab markers of NEC. Mechanical ventilation can lead to thrombocytopenia due to mechanical reasons, shearing effect on the pulmonary vasculature producing increasing platelet consumption [50]. There was a significant association between mechanical ventilation (p<0.004). 35% of the neonates in the severe thrombocytopenia group were mechanically ventilated at one time or the other. This association has been documented by other studies done in this regard [57]. Whether mechanical ventilation itself leads to thrombocytopenia or the underlying condition, that produced respiratory failure, did so; can only be assessed by a more detailed study where daily platelet counts are done before during, and after weaning off from ventilation. Other factors such as hypoxia, hyperbilirubinemia, low Apgar scores,exchange transfusion, and RDS were not significantly associated with neonatal thrombocytopenia. Probably if continuous pulseoximetry was done there would have been an association between hypoxia and neonatal thrombocytopenia, but in our study, all cases did not receive continuous pulse-oximetry. Exchange transfusion done with nonfresh whole blood is known to be associated with neonatal thrombocytopenia53. But only fresh whole blood was used in our study for this purpose, this might be the reason behind there being no association between thrombocytopenia and exchange transfusion in our study. Clinical features: Mucosal bleeding was significantly associated with thrombocytopenia (p=0.002). While 50% of the severely thrombocytopenic neonates had mucosal bleeding only 15.7% and 19.6% of the other two groups bled. The types of bleeding included G.I bleeding, bleeding from the E.T. tube (pulmonary hemorrhage), and bleeding from the oral cavity. The average platelet count of the bleeding neonate was, 1.16×105/µl. It was more than 50,000/µl probably because neonates bleeding due to causes other than quantitative platelet deficiencies such as VKDB were also included. But still, the association between the platelet count and mucosal bleeding was significant (p<0.001). Investigations to rule out IC bleed, neurosonogram, and CT brain, were done only in 27 cases of the 140. Among these cases, the majority (56.8%) were severely thrombocytopenic. Hence there was as a strong correlation between IC bleed and severe thrombocytopenia (p<0.0l). In our study neurodiagnostic tests were done only in the event of a suspicion of intracranial pathology. The incidence of petechiae and purpura was significantly associated with severe thrombocytopenia (p<0.001) with 45% of these neonates having them. This association has been well-reported and documented in the past [38].
The most common symptom other than bleeding was “not feeding well”. But this symptom is a nonspecific one that can be associated with any sick neonate. The most common sign other than bleeding in the severely thrombocytopenic group was delayed capillary refill (>3 sec.).This association might either be due to shock in sick, especially septicemic neonates, who are known to have severe thrombocytopenia, or might be due to excessive blood loss81. But we couldn’t document hypotension due to the unavailability of continuous intra-arterial or oscillometric BP monitoring. Neurological depression was the most common sign in the no thrombocytopenia and the mild to moderate thrombocytopenia group, this association might be due to the increased prevalence of perinatal asphyxia in these groups. The mortality rate was very high, 37%, among the severely thrombocytopenic neonates while it was only 3.72% and 3.92% respectively in the mild to moderate and no thrombocytopenia groups. The proportion of an “Unsatisfactory” outcome was more (80.39%) in the mild to moderate thrombocytopenia group while it was 30 and 52.77% in the severe thrombocytopenia and no thrombocytopenia group. Hence a poor immediate outcome was associated with thrombocytopenia. This association might be due to the higher degree of severity of the underlying illness or due to an increased susceptibility of the neonates to complications, in the severely thrombocytopenic group. The proportion of poor neurodevelopmental outcomes was significantly higher (31.57%) among the severely thrombocytopenic neonates compared to the other two groups. If severe thrombocytopenia itself was responsible for the poor outcome then all the neonates with severe thrombocytopenia and poor outcome should have had IC bleed. But 33.3% of neonates with both severe thrombocytopenia and the poor outcome did not have radiological evidence of IC bleed. So probably the underlying pathology, that produced a low platelet count, might have also affected the developing brain detrimentally. Hence severe thrombocytopenia might just be a marker of the severity of the underlying illness that leads to the poor outcome, rather than being its direct cause. To know whether the low platelet count is in itself an independent risk factor for poor outcome, in other words, whether severe thrombocytopenia can be used as a prognostic indicator in sick neonates; certain statistical methods were adopted. First, by univariate analysis, it was found that 12 variables, Intrauterine growth retardation, a delayed capillary refill at admission, presence or absence of congenital anomalies, mechanical ventilation, exaggerated physiological jaundice, mode of delivery, DIC, and refractory seizures were associated with poor outcome. These variables along with those that are known to influence the outcome of NICU graduates, such as age at presentation, gestational age, perinatal asphyxia, Meconium aspiration syndrome, hyaline membrane disease, NEC, Suspected /proven septicemia and duration of stay, were subjected to multiple logistic regressional analysis. It was found that 6 factors were associated with the outcome independently and one among them was platelet count (p<0.001).
This association just shows us that severe thrombocytopenia is an independent risk factor for poor outcomes. It does not assert that the low platelet count per se was responsible for the poor outcome. It could have been either the low platelet count itself or the many underlying illnesses that presented with severe thrombocytopenia that had directly led to this kind of outcome. But one probable conclusion that can be drawn is, whatever might be the underlying illness, severe thrombocytopenia in a sick neonate is suggestive of a severe form of that illness and that the probability that the neonate will have a poor outcome is high. It can be stated that severe thrombocytopenia, in sick neonates in the NICU, is a poor prognostic indicator. The efficacy of the treatment protocol practiced was assessed based on the percentage increment in platelet count after 24 hours of intervention. It was found that though platelet transfusion produced a higher increment in platelet count compared to fresh whole blood transfusion, the discrepancy was not statistically significant. It can be concluded that though fresh whole blood transfusion might not be as good an option as platelet transfusion in severe thrombocytopenia, it is a good alternative to platelet concentrates in times of its unavailability.
Summary
• Neonatal thrombocytopenia is one of the most common hematological abnormalities in our NICU.
• The prevalence of thrombocytopeniawas 25.45% and severe thrombocytopenia was 08.5% in our NICU.
• The most common etiological association withthrombocytopenia was septicemia.
• Maternal PIH was significantly associated with neonatal thrombocytopenia.
• Neonatal factors associated with neonatal thrombocytopenia, especially severe thrombocytopenia were age at presentation, septicemia, NC, NEC, and assisted ventilation.
• Gestational age, IUGR, and perinatal asphyxia were not associated with neonatal thrombocytopenia.
• Exchange transfusion was not associated with thrombocytopenia, probably due to fresh whole blood use.
• The clinical signs and symptoms associated with neonatal thrombocytopenia, especially severe thrombocytopenia, were bleeding, purpura, and delayed capillary refill.
• The course in the hospital of neonatal thrombocytopenia, especially severe thrombocytopenia, was relatively longer involving a longer period of supplemental oxygen and IV fluids.
• The mortality rate among severely thrombocytopenic neonates was significantly higher (37%).
• Mild to moderate thrombocytopenic neonates had more (80.64%) non-satisfactory immediate outcomes.
• Poor neurodevelopmental outcome at 3 months was significantly associated with severe neonatal thrombocytopenia. With 43.70% of severe thrombocytopenia cases having such an outcome.
• Low platelet count was an independent risk factor for poor outcomes in our study. Hence it could be used as a prognostic indicator in NICU graduates.
• Fresh whole blood transfusion was shown to be a good alternative to platelet transfusion in the treatment of severe neonatal thrombocytopenia as there was no statistically significant difference in the platelet increment after 24 hours between both these procedures.
Conclusion
It was found that the prevalence of thrombocytopenia was high (25.45%) and that of severe thrombocytopenia was 8.5%. Septicemia was the major etiology associated with both severe and mild to moderate thrombocytopenia. The predisposing factors associated with neonatal thrombocytopenia were Maternal PIH, age at presentation, septicemia, NEC, DIC, and assisted ventilation. Glaringly, IUGR, prematurity, and perinatal asphyxia were not associated with neonatal thrombocytopenia in our study. Bleeding of any sort either mucosal, cutaneous, or intra cranial was significantly associated with severe thrombocytopenia. Delayed capillary refill was the most common sign other than bleeding associated with thrombocytopenia. Mortality rate (39%) and poor neurodevelopmental outcome (31.57%) were far more common in the severe thrombocytopenic group. Moreover, low platelet count was found to be an independent risk factor for poor outcomes in our NICU graduates. Hence it can be concluded that thrombocytopenia is very much common among our NICU admissions. Septicemia is its most important and most common cause. Various maternal and neonatal factors can be associated with thrombocytopenia. Severe thrombocytopenic neonates bleed more frequently and can have unstable vital signs such as poor perfusion at presentation. Poor outcomes both immediate and short term are very much associated with severe thrombocytopenia at presentation. The most significant conclusion of our study was that severe thrombocytopenia can be used as a prognostic indicator in sick neonates. But to generalize this statement, and apply it to all neonatal admissions, more studies are required in this regard with similar results. Fresh whole blood transfusion is a good alternative to platelet concentrates in the treatment of severe thrombocytopenia.
Declarations
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Roberts, I., and N. A. Murray. "Neonatal thrombocytopenia: causes and management." Archives of Disease in Childhood-Fetal and Neonatal Edition, Vol. 88, No. 5, 2003, pp. 359-64.
Google Scholar Crossref - Roberts, Irene AG, and Neil A. Murray. "Neonatal thrombocytopenia: new insights into pathogenesis and implications for clinical management." Current opinion in paediatrics, Vol. 13, No. 1, 2001, pp. 16-21.
Google Scholar - Sola, Martha C., Antonio Del Vecchio, and Lisa M. Rimsza. "Evaluation and treatment of thrombocytopenia in the neonatal intensive care unit." Clinics in perinatology, Vol. 27, No. 3, 2000, pp. 655-79.
Google Scholar Crossref - Tirupathi, Keerthi, Keerti Swarnkar, and Jayant Vagha. "Study of risk factors of neonatal thrombocytopenia." International Journal of Contemporary Pediatrics, Vol. 1, 2017, pp. 191-96.
Google Scholar Crossref - Niparko, John K., and Yuri Agrawal. "The epidemiology of hearing loss: how prevalent is hearing loss." Cochlear implants: Principles and practices, 2000, pp. 88-92.
Google Scholar - Raizada, N., et al. "Neonatal thrombocytopenia due to pregnancy induced hypertension." The Indian Journal of Pediatrics, Vol. 63, No. 2, 1996, pp. 226-28.
Google Scholar - Israels, Sara J., Margaret L. Rand, and Alan D. Michelson. "Neonatal platelet function." Seminars in thrombosis and hemostasis. Vol. 29. No. 4.
Google Scholar Crossref - Nathal, D. G., and H. Stuart. "Oski’s Hematology Of Infancy & Childhood." 6th edn, Philadelphia, Saunders 2003.
- Murray, Neil A., and Irene AG Roberts. "Circulating megakaryocytes and their progenitors in early thrombocytopenia in preterm neonates." Pediatric research, Vol. 40, No. 1, 1996, pp. 112-19.
Google Scholar Crossref - ÜSTÜN, Nuran. "Effect of perinatal factors on platelet indices in late preterm and term newborns." Journal of Contemporary Medicine, Vol.11, No. 5, pp. 661-65.
Google Scholar Crossref - Dame, C. "Developmental biology of thrombopoietin in the human fetus and neonate." Acta Paediatrica, Vol. 91, 2002, pp. 54-65.
Google Scholar Crossref - Saxonhouse, Matthew A., et al. "Reticulated platelet percentages in term and preterm neonates." Journal of Pediatric Hematology/Oncology, Vol. 26, No. 12, 2004, pp. 797-802.
Google Scholar - Forestier, François, et al. "Developmental hematopoiesis in normal human fetal blood.", 1991, pp. 2360-63.
Google Scholar Crossref - McCloy, Mary P., et al. "Interleukin-11 levels in healthy and thrombocytopenic neonates." Pediatric research, Vol. 51, No. 6, 2002, pp. 756-60.
Google Scholar Crossref - Castle, Valerie, et al. "Frequency and mechanism of neonatal thrombocytopenia." The Journal of paediatrics, Vol. 108, No. 5, 1986, pp. 749-55.
Google Scholar Crossref - Roberts, Irene AG, and Neil A. Murray. "Thrombocytopenia in the newborn." Current opinion in paediatrics, Vol. 15, No. 1, 2003, 17-23.
Google Scholar - Uhrynowska, Malgorzata, Krystyna Maslanka, and Barbara Zupanska. "Neonatal thrombocytopenia: incidence, serological and clinical observations." American journal of perinatology, Vol. 14, No. 7, 1997, pp. 415-18.
Google Scholar Crossref - Sainio, Susanna, et al. "Thrombocytopenia in term infants: a population-based study." Obstetrics and Gynecology, Vol. 95, No. 3, 2000, pp. 441-46.
Google Scholar Crossref - Ören, Hale, et al. "Assessment of clinical impact and predisposing factors for neonatal thrombocytopenia." The Indian Journal of Pediatrics, Vol. 61, No. 5, 1994, pp. 551-58.
Google Scholar Crossref - Andrew, Maureen, et al. "Clinical impact of neonatal thrombocytopenia." The Journal of paediatrics, Vol. 110, No. 3, 1987, pp. 457-64.
Google Scholar Crossref - Beiner, Mario E., et al. "Risk factors for neonatal thrombocytopenia in preterm infants." American journal of perinatology, Vol. 20, No. 1, 2003, pp. 49-54.
Google Scholar Crossref - Mehta, Paulette, et al. "Thrombocytopenia in the high-risk infant." The Journal of paediatrics, Vol. 97, No. 5, 1980, pp. 791-94.
Google Scholar Crossref - Morales, WALTER J., and M. I. C. H. A. E. L. Stroup. "Intracranial hemorrhage in utero due to isoimmune neonatal thrombocytopenia." Obstetrics and gynecology, Vol. 65, No. 3, 1985, pp. 20-21.
Google Scholar - Sola, M. C., and L. M. Rimsza. "Mechanisms underlying thrombocytopenia in the neonatal intensive care unit." Acta Pædiatrica, Vol. 91, 2002, pp. 66-73.
Google Scholar Crossref - Peterec, Steven M., et al. "Reticulated platelet values in normal and thrombocytopenic neonates." The Journal of paediatrics, Vol. 129, No. 2, 1996, pp. 269-74.
Google Scholar Crossref - Slayton, William B., et al. "Developmental differences in megakaryocyte maturation are determined by the microenvironment." Stem cells, Vol. 23, No. 9, 2005, pp. 1400-08.
Google Scholar Crossref - Saxonhouse, Matthew A., et al. "Effects of anoxia on megakaryocyte progenitors derived from cord blood CD34pos cells." European journal of haematology, Vol. 71, No. 5, 2003, pp. 359-65.
Google Scholar Crossref - Hord, Jeffrey D., James C. Gay, and James A. Whitlock. "Thrombocytopenia in neonates with trisomy 21." Archives of pediatrics and adolescent medicine, Vol. 149, No. 7, 1995, pp. 824-25.
Google Scholar Crossref - Markenson, Alicejane Lippner, HILGARTNER MW, and MILLER DR. "Transient thrombocytopenia in 18-trisomy.", 1975.
Google Scholar - Kato, Kazunobu, et al. "Genetic deletion of mouse platelet glycoprotein Ibβ produces a Bernard-Soulier phenotype with increased α-granule size." Blood, Vol. 104, No. 8, 2004, pp. 2339-44.
Google Scholar Crossref - Sola, Martha C., et al. "A neonate with severe thrombocytopenia and radio-ulnar synostosis." Journal of perinatology, Vol. 24, No. 8, 2004, pp. 528-30.
Google Scholar - Dong, Fan, et al. "Genotype–phenotype correlation in MYH9‐related thrombocytopenia." British journal of haematology, Vol. 130, No. 4, 2005, pp. 620-27.
Google Scholar Crossref - Wenger, Sharon L., et al. "Molecular characterization of an 11q interstitial deletion in a patient with the clinical features of Jacobsen syndrome." American Journal of Medical Genetics, Vol. 140, No. 7, 2006, pp. 704-08.
Google Scholar Crossref - Raslova, Hana, et al. "FLI1 monoallelic expression combined with its hemizygous loss underlies Paris-Trousseau/Jacobsen thrombopenia." The Journal of clinical investigation, Vol. 114, No. 1, 2004, pp. 77-84.
Google Scholar - Grossfeld, Paul D., et al. "The 11q terminal deletion disorder: a prospective study of 110 cases." American journal of medical genetics, Vol. 129, No. 1, 2004, pp. 51-61.
Google Scholar Crossref - Geddis, Amy E., and Kenneth Kaushansky. "Inherited thrombocytopenias: toward a molecular understanding of disorders of platelet production." Current opinion in paediatrics, Vol. 16, No. 1, 2004, pp. 15-22.
Google Scholar - Homans, Alan. "Thrombocytopenia in the neonate." Pediatric Clinics, Vol. 43, No. 3, 1996, pp. 737-56.
Google Scholar Crossref - Arkhangel'skiĭ, A. V., and G. N. Masliakova. "Frequency and morphology of DIC-syndrome in children in early neonatal period." Arkhiv Patologii, Vol. 58, No. 5, 1996, pp. 61-63.
Google Scholar - Guida, Jack D., et al. "Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response?." Pediatrics, Vol. 111, No. 6, 2003, pp. 1411-15.
Google Scholar Crossref - Zipursky, Alvin, and Husni M. Jaber. "The haematology of bacterial infection in newborn infants." Clinics in Haematology, Vol. 7, No. 1, 1978, pp. 175-93.
Google Scholar Crossref - Padovani, E. M., et al. "Sepsis caused by Candida in the neonatal period." La Pediatria medica e chirurgica: Medical and surgical paediatrics, Vol. 19, No. 2, 1997, pp. 83-88.
Google Scholar - Melville, C., et al. "Early onset systemicCandida infection in extremely preterm neonates." European journal of paediatrics, Vol. 155, No. 10, 1996, pp. 904-06.
Google Scholar Crossref - Surmont, I., et al. "Malassezia furfur fungaemia in infants receiving intravenous lipid emulsions. A rarity or just underestimated?" European journal of paediatrics, Vol. 148, No. 5, 1989, pp. 435-38.
Google Scholar Crossref - Sridhar, S., and K. A. Kuruvilla. "Kasabach-Merrltt syndrome." Indian Pediatrics, Vol. 42, No. 10, 2005, p. 1045.
Google Scholar - Ontachi Y, Asakura H, Omote M, Yoshida T, Matsui O. “Kasabach Merritt syn with giant liver hemangioma: effect of combined therapy with danaproid sodium and tranexamic acid”. Haematologica 2005.
Crossref - Loirat C, et al. “Thrombotic thrombocytopenic purpura associated with vWfactor-cleaving protease (ADAMTSI3) deficiency in children”. Semin Thromb Hemost, Vol. 32, No. 2, 2006, pp. 90-7.
Crossref - Nurden, Paquita, et al. "Platelet ultrastructural abnormalities in three patients with type 2B von Willebrand disease." British journal of haematology, Vol. 110, No. 3, 2000, pp. 704-14.
Google Scholar Crossref - Chou, Lin-Lin, Chih-Ping Chang, and Lie-Chou Wu. "Neonatal coxsackievirus B1 infection associated with severe hepatitis: report of three cases." Zhonghua Minguo Xiao er ke yi xue hui za zhi, Vol. 36, No. 4, 1995, pp. 296-99.
Google Scholar - Sabri, Siham, et al. "Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment." Blood, Vol. 108, No. 1, 2006, pp. 134-40.
Google Scholar Crossref - Sutor, Anton H., Anthony KC Chan, and Patricia Massicotte. "Low-molecular-weight heparin in pediatric patients." Seminars in thrombosis and hemostasis. Vol. 30. No. 1, 2004.
Google Scholar Crossref - Kumar, R. K., and V. Y. H. Yu. "Prolonged low‐dose indomethacin therapy for patent ductus arteriosus in very low birthweight infants." Journal of paediatrics and child health, Vol. 33, No. 1, 1997, pp. 38-41.
Google Scholar Crossref - Patra, Kousiki, et al. "Adverse events associated with neonatal exchange transfusion in the 1990s." The Journal of paediatrics, Vol. 144, No. 5, 2004, pp. 626-31.
Google Scholar Crossref - Shibata, Junpei, et al. "Hemostasis and coagulation at a hematocrit level of 0.85: functional consequences of erythrocytosis." Blood, Vol. 101, No. 11, 2003, pp. 4416-22.
Google Scholar Crossref - Burrows, R. F., and M. Andrew. "Neonatal thrombocytopenia in the hypertensive disorders of pregnancy." Obstetrics and gynecology, Vol. 76, No. 2, 1990, pp. 234-38.
Google Scholar - Aman, Ijaz, Khawaja A. Hassan, and Tahir M. Ahmad. "The study of thrombocytopenia in sick neonates." Journal of the College of Physicians and Surgeons—pakistan, Vol. 14, No. 5, 2004, pp. 282-85.
Google Scholar Crossref - Kahn, Doron J., Douglas K. Richardson, and Henny H. Billett. "Association of thrombocytopenia and delivery method with intraventricular hemorrhage among very-low-birth-weight infants." American journal of obstetrics and gynecology, Vol. 186, No. 1, 2002, pp. 109-16.
Google Scholar Crossref - Andrew, M., et al. "A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants." The Journal of paediatrics, Vol. 123, No. 2, 1993, pp. 285-91.
Google Scholar Crossref - Blanchette, V. S., J. Johnson, and M. Rand. "The management of alloimmune neonatal thrombocytopenia." Best Practice and Research Clinical Haematology, Vol. 13, No. 3, 2000, pp. 365-90.
Google Scholar Crossref - Bussel, J., C. Kaplan, and J. McFarland. "Recommendations for the evaluation and treatment of neonatal autoimmune and alloimmune thrombocytopenia." Thrombosis and haemostasis, Vol. 65, No. 5, 1991, pp. 631-34.
Google Scholar Crossref - Kaplan, Dr Cécile. "Platelet alloimmunity: the fetal/neonatal alloimmune thrombocytopenia." Vox sanguinis, Vol. 83, 2002, pp. 289-91.
Google Scholar Crossref - Veerareddy, Sukrutha, and Pranav P. Pandya. "Thrombocytopenia in Pregnancy: Fetal and Neonatal Alloimmune Thrombocytopenia." Disorders of Thrombosis and Hemostasis in Pregnancy, 2015. pp. 279-94.
Google Scholar Crossref - Radder, Celine M., Anneke Brand, and Humphrey HH Kanhai. "A less invasive treatment strategy to prevent intracranial hemorrhage in fetal and neonatal alloimmune thrombocytopenia." American journal of obstetrics and gynecology, Vol. 185, No. 3, 2001, pp. 683-88.
Google Scholar Crossref - Johnson JA, Ryan G,. “Prenatal diagnosis and management of neonatal alloimmune thrombocytopenia”. Semin Perinatol, Vol. 21, No. 1, 1997, pp. 45-52.
Crossref - Cook, ROBERT L., et al. "Immune thrombocytopenic purpura in pregnancy: a reappraisal of management." Obstetrics and Gynecology, Vol. 78, No. 4, 1991, pp. 578-83.
Google Scholar - Murray, N. A., et al. "Platelet transfusion in the management of severe thrombocytopenia in neonatal intensive care unit patients." Transfusion medicine, Vol. 12, No. 1, 2002, pp. 35-41.
Google Scholar Crossref - Murray NA, Roberts IA. “Neonatal transfusion practice”. Archives of Disease in Childhood: Fetal and Neonatal Edition, 2004, Vol. 89, No. 2, 2004, pp. 101-07.
Crossref - Inagaki, Koji, et al. "Induction of megakaryocytopoiesis and thrombocytopoiesis by JTZ-132, a novel small molecule with thrombopoietin mimetic activities." Blood, Vol. 104, No. 1, 2004, pp. 58-64.
Google Scholar Crossref - Agarwal, D. K., and K. N. Agarwal. "Physical growth in Indian affluent children (birth-6 years)." Indian paediatrics, Vol. 31, 1994, p. 377.
Google Scholar - Glascoe, Frances Page, et al. "Accuracy of the Denver-II in developmental screening." Pediatrics, Vol. 89, No. 6, 1992, pp. 1221-25.
Google Scholar Crossref - Frankenburg, William K., et al. "The Denver II: a major revision and restandardization of the Denver Developmental Screening Test." Pediatrics, Vol. 89, No. 1, 1992, pp. 91-97.
Google Scholar Crossref - Kuruvilla, Kurien A., et al. "Bacterial profile of sepsis in a neonatal unit in south India." Indian Pediatrics, Vol. 35, No. 9, 1998, pp. 851-58.
Google Scholar - Lubchenco, Lula O., et al. "Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation." Pediatrics, Vol. 32, No. 5, 1963, pp. 793-800.
Google Scholar Crossref - Faust, Saul N., Robert S. Heyderman, and Michael Levin. "Disseminated intravascular coagulation and purpura fulminans secondary to infection." Best Practice and Research Clinical Haematology, Vol. 13, No. 2, 2000, pp. 179-97.
Google Scholar Crossref - Kim, Brian Y., Jay Kang, and Kwang Sik-Kim. "Invasion processes of pathogenic Escherichia coli." International journal of medical microbiology, Vol. 295, No. 6, 2005, pp. 463-70.
Google Scholar Crossref - Engle, William D., Abbot R. Laptook, and Jeffrey M. Perlman. "Acute changes in arterial carbon dioxide tension and acid–base status and early neurologic characteristics in term infants following perinatal asphyxia." Resuscitation, Vol. 42, No. 1, 1999, pp. 11-17.
Google Scholar Crossref - Faust, Saul N., Robert S. Heyderman, and Michael Levin. "Disseminated intravascular coagulation and purpura fulminans secondary to infection." Best Practice and Research Clinical Haematology, Vol. 13, No. 2, 2000, pp. 179-97.
Google Scholar Crossref - Kim, Brian Y., Jay Kang, and Kwang Sik Kim. "Invasion processes of pathogenic Escherichia coli." International journal of medical microbiology, Vol. 295, No. 6, 2005, pp. 463-70.
Google Scholar Crossref - Engle, William D., Abbot R. Laptook, and Jeffrey M. Perlman. "Acute changes in arterial carbon dioxide tension and acid–base status and early neurologic characteristics in term infants following perinatal asphyxia." Resuscitation, Vol. 42, No. 1, 1999, pp. 11-17.
Google Scholar Crossref - Gonzalez de Dios, J. "Definition of perinatal asphyxia in medical literature: the need of a consensus." Revista de neurologia, Vol. 35, No. 7, 2002, pp. 628-34.
Google Scholar