Research - International Journal of Medical Research & Health Sciences ( 2020) Volume 9, Issue 12
Clinicopathological study of vesiculobullous diseases with special emphasis on autoimmune disorders-two year study in a resource setting
Sherin Daniel*, Nandakumar G, Krishna G Balachandran Nair and Santha SadasivanSherin Daniel, Department of Pathology, Government Medical College, Trivandrum, Kerala, India, Email: sherin1607@gmail.com
Received: 12-Nov-2020 Accepted Date: Dec 21, 2020 ; Published: 28-Dec-2020
Abstract
Objective: To identify the characteristic histopathologic findings and analyse the diagnostic concordance between clinical and histopathological diagnosis of patients with vesiculobullous diseases. Methods: This was a two-year descriptive study conducted in the Department of Pathology where all skin biopsies diagnosed as vesiculobullous diseases were included. A detailed history and relevant clinical examination findings were documented. The specimens were formalin-fixed, paraffin-embedded and stained with haematoxylin and eosin. Direct immunofluorescence was advised for patients where histopathologic features were inconclusive. Results: There were 111 skin biopsy specimens included in the study. Demographic details showed female predominance (65.8%) and the most frequent age group being (40-69) years (29%). Generalised distribution of the lesions over the trunk (53%) associated with itching was the common clinical manifestation. Most frequent clinical diagnosis in the study was pemphigus vulgaris (38%) and bullous pemphigoid (31%). Histopathologic findings revealed subepidermal blister (52.3%) as the most common finding. Inflammatory cell infiltrates constituted the main content of the blister (44.1%) with eosinophils being the predominant cell type (37.8%). There was a good positive correlation of 0.546 between histopathologic and clinical diagnosis. Conclusion: Bullous pemphigoid constituted the most common vesiculobullous disease followed by pemphigus vulgaris in this study. Histopathological diagnosis correlated well with clinical diagnosis in most of the vesiculobullous disease, emphasizing the need for skin biopsy in all cases of vesiculobullous diseases. Direct Immunofluorescence is a helpful adjunct in scenarios where clinical and/ or histopathological features are inconclusive.
Keywords
Blistering diseases, Autoimmune, Tzanck smear, Histopathology, Level of the split, Eosinophils, Direct immunofluorescence
Introduction
Blistering diseases are a heterogenous group of disorders with protean manifestations and the reaction pattern is characterised by the presence of vesicles or bulla at any level within the epidermis or the dermo-epidermal junction [1]. Vesiculobullous diseases have a history as old as that of medicine. Although blisters have drawn great attention to caregivers in the ancient period, only modern times have seen the origin of a clear classification of these diseases based upon the clinical, histopathologic and immunofluorescence findings. There are five principle ways that can lead to a blister formation, of these, immunological reaction accounts for one of the most important group of diseases producing blisters [2]. Autoimmune blistering diseases are characterized by the presence of pathogenic autoantibodies targeting various adhesion molecules of the skin which lead to the formation of blister. These autoimmune blistering diseases have a dramatic clinical presentation and are associated with substantial morbidity and mortality. These diseases have been the subject of intensive investigation in the recent years [3]. Very often, these diseases cannot be differentiated clinically and hence requires the help of histopathological findings for diagnosis. Biopsy is the gold standard for the diagnosis of vesiculobullous lesions. They also provide added information regarding the pathogenic mechanisms behind these lesions [4]. Immunofluorescence (IF) studies and Electron Microscopy (EM) assist in the diagnosis of cases where histopathology is not conclusive [5]. Recent advances in investigative dermatology have created new horizons, techniques such as immunoblotting and immune electron microscopy may refine the diagnosis in individual patient.
However, these investigations are available only in advanced research laboratories. Various studies on these diseases have highlighted only a particular entity or some specific aspect of it, but a detailed clinical and histopathologic study have been attempted only by very few people, especially in this part of India. The accurate diagnosis of vesiculobullous diseases of the skin requires close attention to the clinical details, histopathological findings supplemented by the confirmation using immunofluorescent techniques wherever possible. This formed the ground for the present study, which undertook to critically evaluate the clinical features and the histopathologic findings of various vesiculobullous disorders of the skin for their diagnostic and therapeutic potential.
Materials and Methods
Objectives of the Study
Primary objective: To identify the characteristic histopathological findings of vesiculobullous diseases.
Secondary objective: To determine the diagnostic concordance between clinical and histopathological diagnosis of patients with vesiculobullous diseases.
Study Design
Descriptive study
Study Setting
Department of Pathology, Government Medical College, Trivandrum, Kerala, South India.
Study Period
Two years (September 2012-August 2014).
Study Sample
One hundred and eleven (111) skin biopsy specimens with a histopathologic diagnosis of vesiculobullous diseases were selected.
Inclusion Criteria
All skin biopsy specimens diagnosed as vesiculobullous disease received in the Department of Pathology.
Exclusion Criteria
Vesiculobullous lesions due to thermal and traumatic causes were excluded.
Ethical Clearance
The present study was carried out in accordance with the Communication of Decision of the Institutional Ethics Committee (IEC)/Institutional Review Board (IRB). Ethical approval for this study was obtained from the Human Ethics Committee, Medical College, Thiruvananthapuram.
Methodology
The study was conducted in the Department of Pathology, Government Medical College, Trivandrum over a period of two years. Detailed history regarding age, gender, presenting complaints and associated symptoms, history of drug or allergic reaction, thermal or traumatic aetiology, distribution and morphology of blisters, presence or absence of mucosal involvement, associated co-morbidities, clinical diagnosis and relevant investigations if done were recorded. Relevant clinical examination findings and investigations were also included. Skin biopsy specimens were received in formalin fixed, paraffin embedded, 4 μm thin sections were taken and stained with haematoxylin and eosin for evaluation. Histomorphology like the level of split, content of the blister, predominant type of cell observed in the blister, associated epidermal and dermal changes were studied. Since immunofluorescence was not available in our institution, in those patients in whom histopathological diagnosis was inconclusive and where patients required an early treatment, the skin biopsy specimens for immunofluorescence confirmation were sent to a higher institution where advanced facilities were available. The findings were then compared with the histopathological features.
Statistical Analysis
All the data obtained were entered in a master sheet, completeness checked, and analysis done with the help of SPSS Software version 20. Data is expressed in its frequency and percentage to elucidate associations and comparisons between different parameters. Chi Square (x²) Test was used as non-parametric test. Comparison of the results was done using Pearson’s correlation.
Results
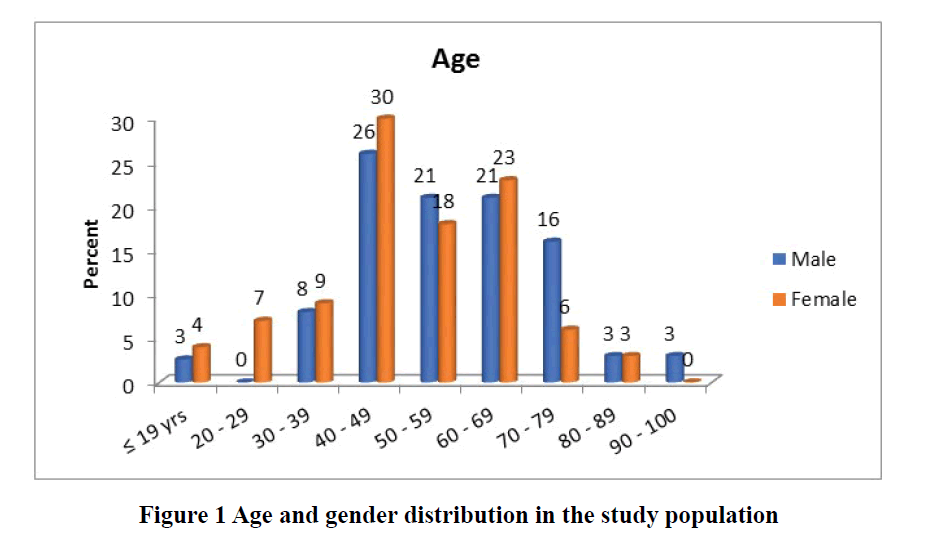
There were 111 skin biopsy specimens diagnosed as vesiculobullous diseases and included in the study over a period of 2 years, which constituted 5.28% of all skin biopsies. This study showed a female preponderance (73 cases; 65.8%) with male: female ratio of 1:1.92. Majority of patients presented between 40-49 years of age (32 cases: 29 %). Youngsters and geriatric population were meagre in the present study. The youngest was a 3-year-old child and the oldest was 95 years (Figure 1). Pemphigus Foliaceous (PF) was seen in a slightly elderly population in the present study. Pemphigus Vulgaris (PV) was the most common disorder in the adolescent population whereas Bullous Pemphigoid (BP) constituted the maximum number of cases in the geriatric population.
The most frequent age group in the present study was in the range of 40-49 years followed by 60-69 years. Mean age was 51.88 ± 32.1 years.
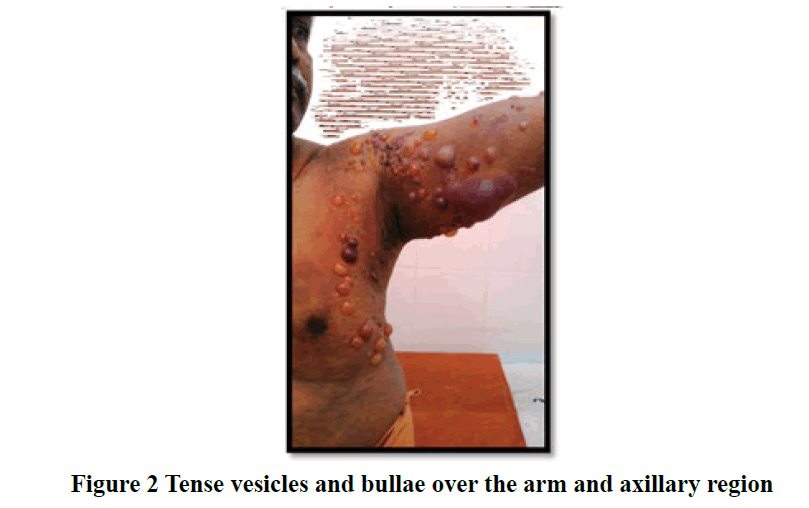
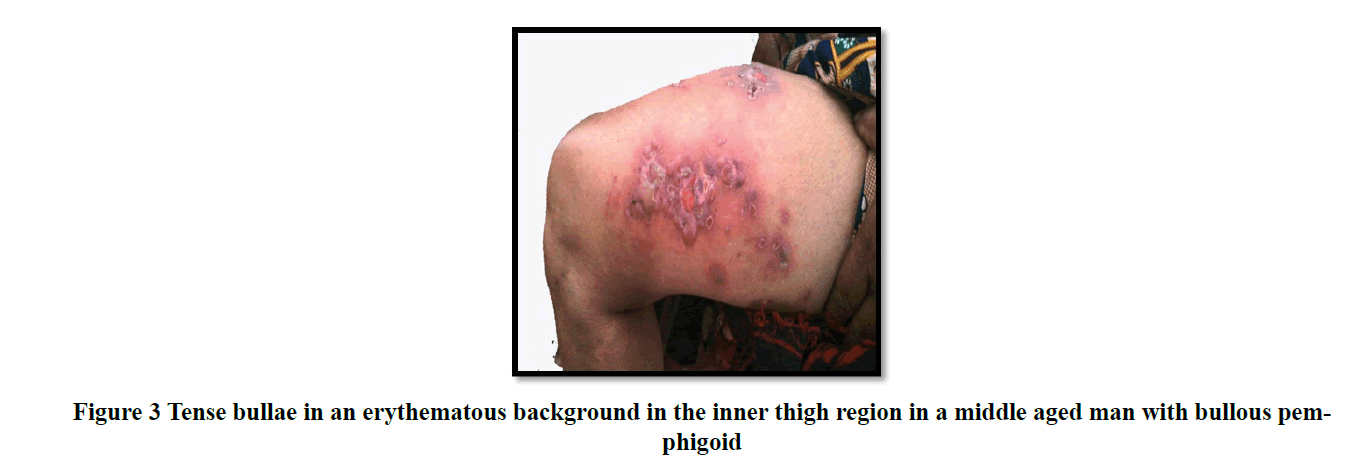
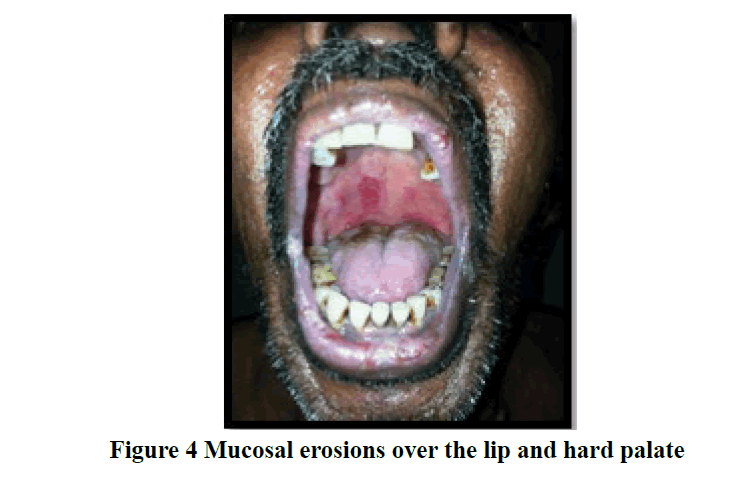
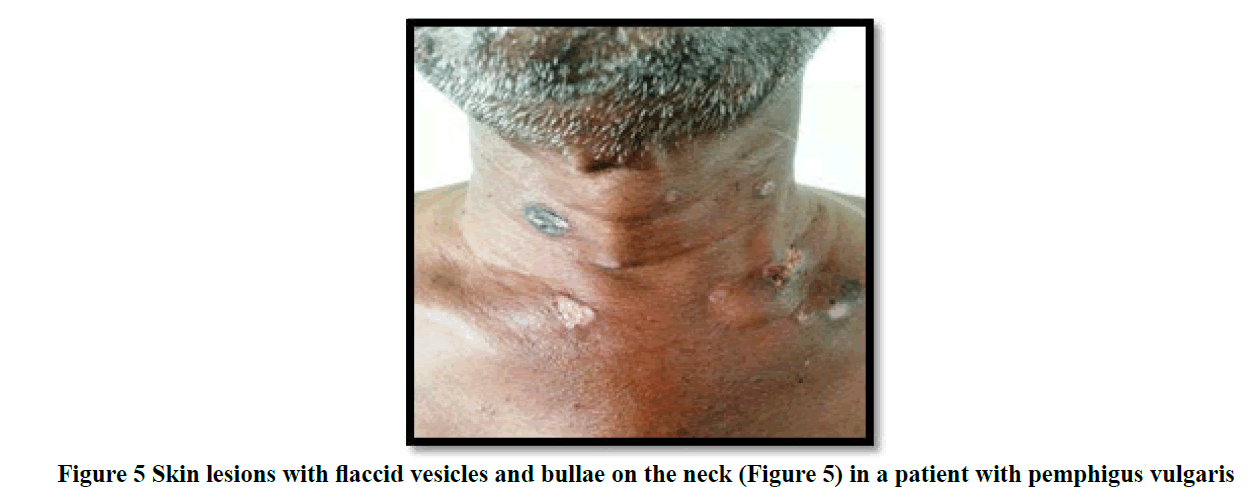
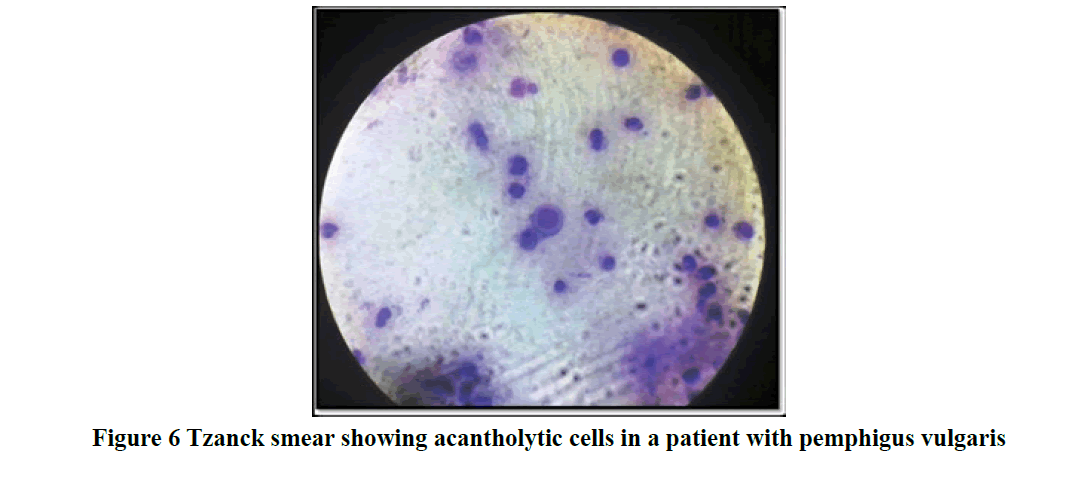
Most patients in the study presented within a duration of one month to one year (46 cases; 41%) with chief clinical symptom of itching associated with vesicles and bullae (61 cases; 55%) on the skin. Mucous membrane lesions were seen in 43% of cases (Table 1) especially in patients with pemphigus vulgaris (Figure 2 and 3). Trunk (21 cases; 19%) was found to be the primary site of onset of the disease followed by oral lesions, extremities, scalp, and face lesions constituting 18% of cases each. Generalised distribution of vesiculobullous lesions was noted in 53% (59/111) of cases in the study. Most of the cases had both flaccid and tense vesicles and bullae (40/97; 36%). Tense bullae were commonly seen in patients with bullous pemphigoid (Figure 4 and 5). Most patients did not have any significant associated co-morbidity. Nikolsky sign was done in 51/111 cases and was positive in 41% patients and bullae spread sign was done 52/111 cases and was positive in 46% patients. Tzanck smear was done in 36/111 cases (32.4%), out of which 27.2% (10/36) showed only acantholytic cells (Figure 6), 50% (18/36) showed only inflammatory cells and 22.2% (8/36) showed both acantholytic and inflammatory cells.
| Associated lesion | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | Frequency | Percent | |
| Vesicles and bullae | 32 | 84 | 65 | 89 | 97 | 87 |
| Normal skin | 28 | 74 | 54 | 74 | 82 | 74 |
| Mucous membrane lesion | 15 | 40 | 33 | 45 | 48 | 43 |
| Crust | 7 | 18 | 25 | 34 | 32 | 29 |
| Erythematous skin | 10 | 26 | 19 | 26 | 29 | 26 |
| Papule | 5 | 13 | 20 | 27 | 25 | 23 |
| Erosions | 7 | 18 | 19 | 26 | 26 | 23 |
| Excoriations | 6 | 16 | 11 | 15 | 17 | 15 |
| Pustule | 0 | 0 | 6 | 8 | 6 | 5 |
Vesicles and bullae were seen in 87% of cases. 74% of cases had normal skin and mucous membrane lesions were seen in 43% of cases. Other lesions like crusts, erosions, papules, pustules were predominant in females than in males and were not prevalent on a higher percentage.
Table 1: Distribution of associated dermatological findings in the study
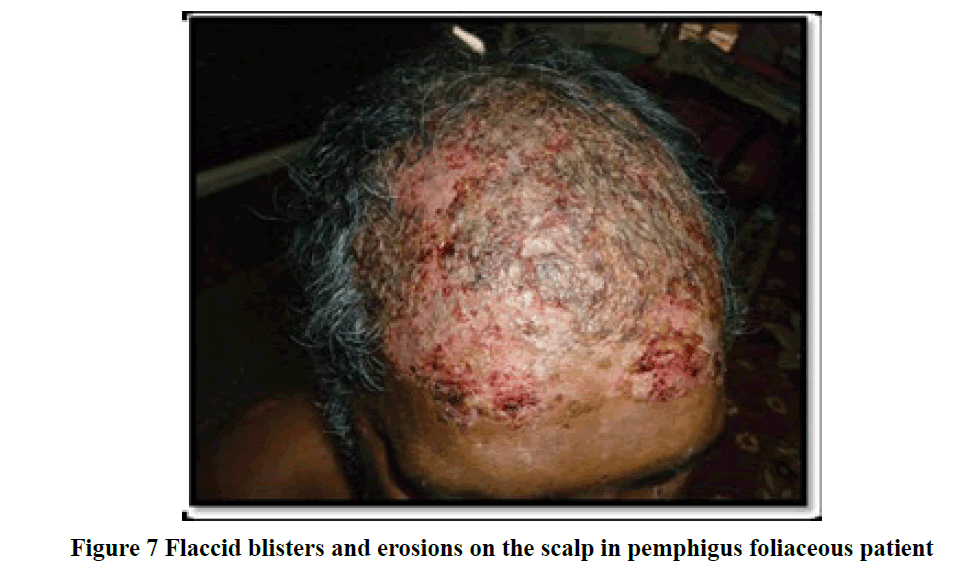
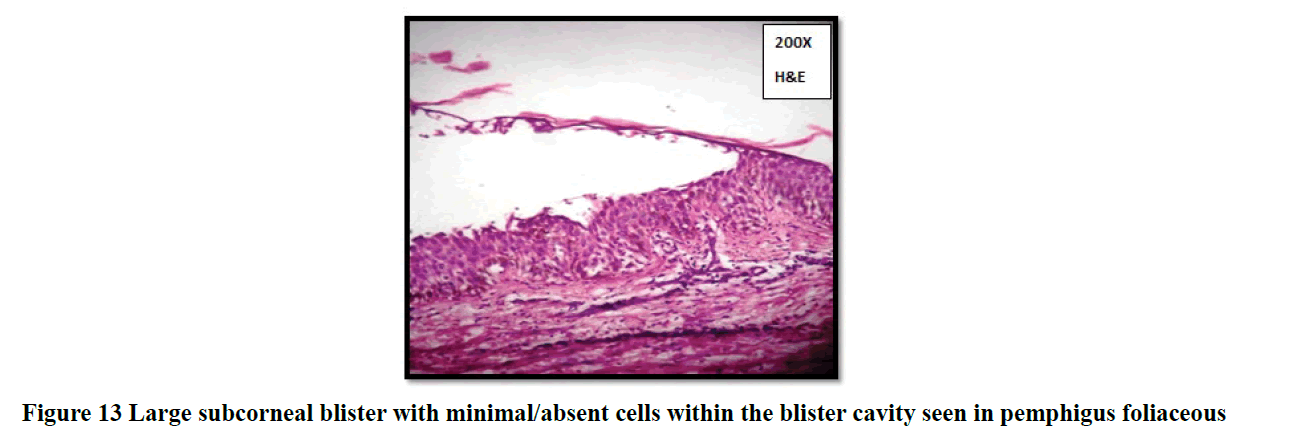
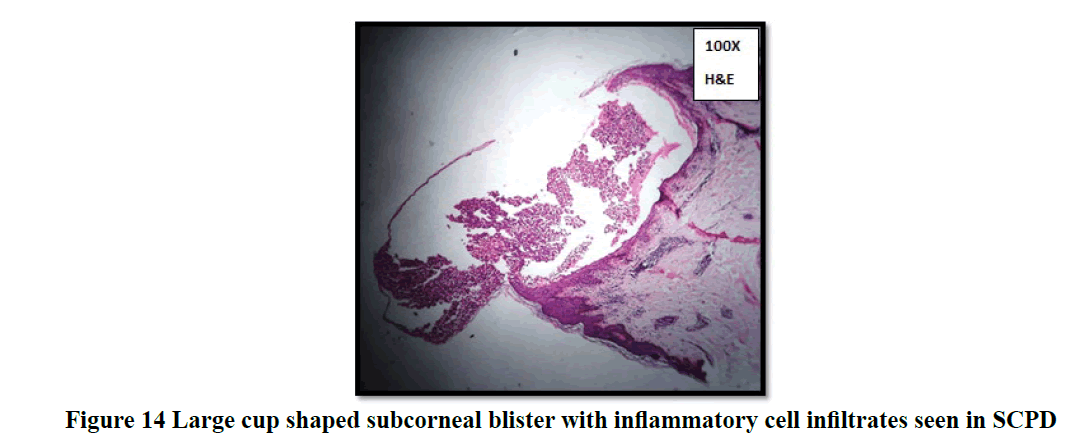
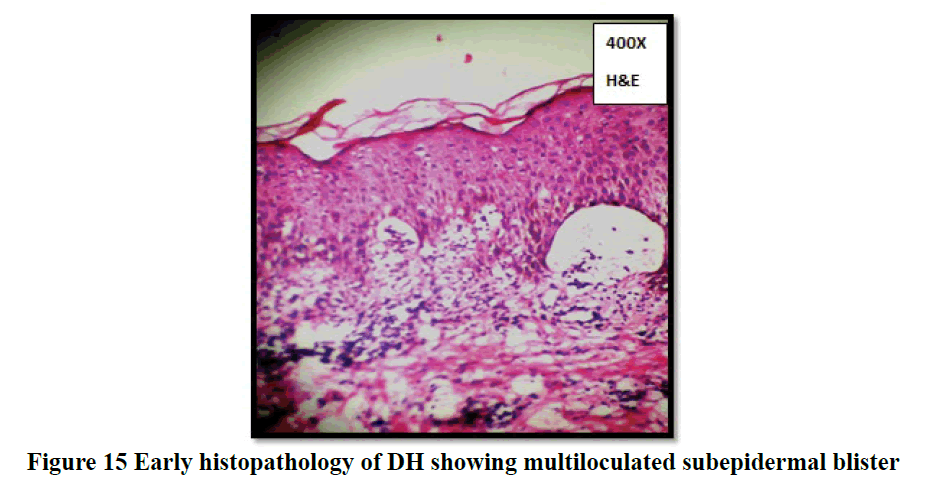
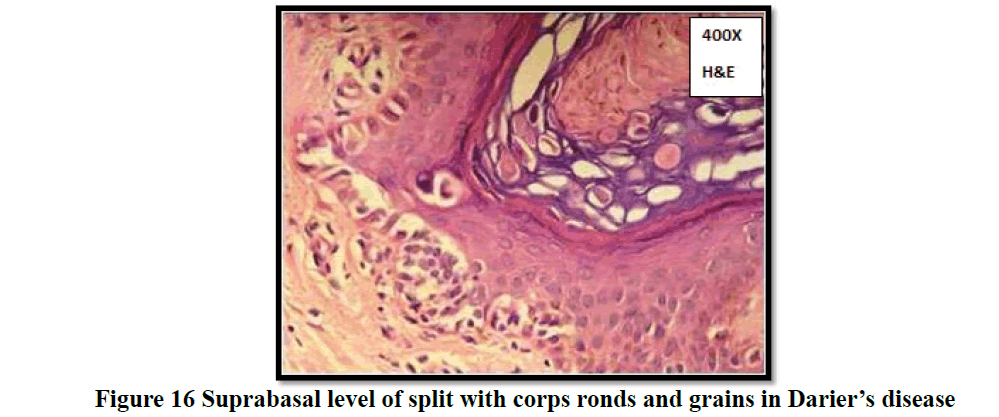
Pemphigus vulgaris (42 cases; 38%) and bullous pemphigoid (34 cases; 31%) were the most frequent clinical diagnosis observed. The rest formed a minor proportion which included 7% of pemphigus foliaceous (Figure 7), 3% of Dermatitis Herpetiformis (DH), Darier disease and bullous lesion of lupus erythematosus each, 2% of Hailey Hailey and Lichen Planus Pemphigoid (LPP) each and 1% of Subcorneal Pustular Dermatosis (SCPD), drug induced, epidermolysis bullosa, linear IgA dermatosis, Paraneoplastic Pemphigus (PNP), mucous membrane pemphigoid and Chronic Bullous Disorder of Childhood (CBDC) each (Table 2).
| Clinical diagnosis | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | Frequency | Percent | |
| Pemphigus vulgaris | 13 | 34 | 29 | 40 | 42 | 38 |
| Bullous pemphigoid | 12 | 32 | 22 | 30 | 34 | 31 |
| Pemphigus foliaceous | 3 | 8 | 5 | 7 | 8 | 7 |
| Dermatitis herpetiformis | 1 | 3 | 3 | 4 | 4 | 3 |
| Darier’s disease | 1 | 3 | 3 | 4 | 4 | 3 |
| Bullous lesion of lupus erythematosus | 1 | 3 | 2 | 3 | 3 | 3 |
| Erythema multiforme | 0 | 0 | 4 | 5 | 4 | 3 |
| Hailey-Hailey disease | 1 | 3 | 1 | 1 | 2 | 2 |
| Lichen planus pemphigoid | 0 | 0 | 2 | 3 | 2 | 2 |
| Sub-corneal pustular dermatosis | 0 | 0 | 1 | 1 | 1 | 1 |
| Drug induced | 1 | 3 | 0 | 0 | 1 | 1 |
| Epidermolysis bullosa | 0 | 0 | 1 | 1 | 1 | 1 |
| Linear IgA dermatosis | 1 | 3 | 0 | 0 | 1 | 1 |
| Paraneoplastic pemphigus | 1 | 3 | 0 | 0 | 1 | 1 |
| Mucous membrane pemphigoid | 1 | 3 | 0 | 0 | 1 | 1 |
| Epidermodysplasia verruciformis | 1 | 3 | 0 | 0 | 1 | 1 |
| CBDC | 1 | 3 | 0 | 0 | 1 | 1 |
Chi square: 0.421; p value >0.05.
Among the various vesiculobullous diseases, a clinical diagnosis of pemphigus vulgaris was arrived in 38% of cases followed by 31% cases of bullous pemphigoid and 7% cases of pemphigus foliaceous. The rest formed a minor proportion.
Table 2: Distribution of various clinical diagnoses in the study
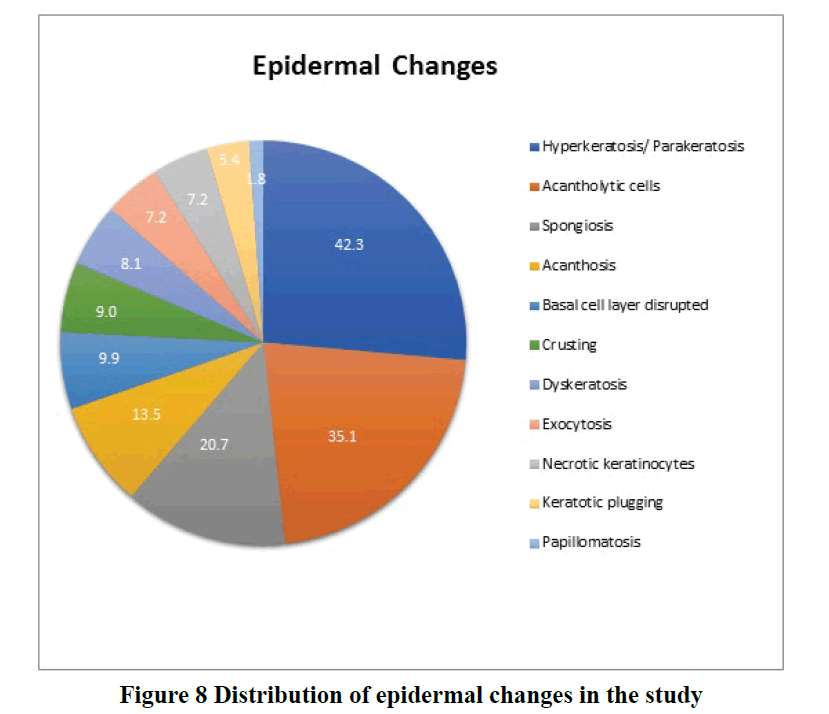
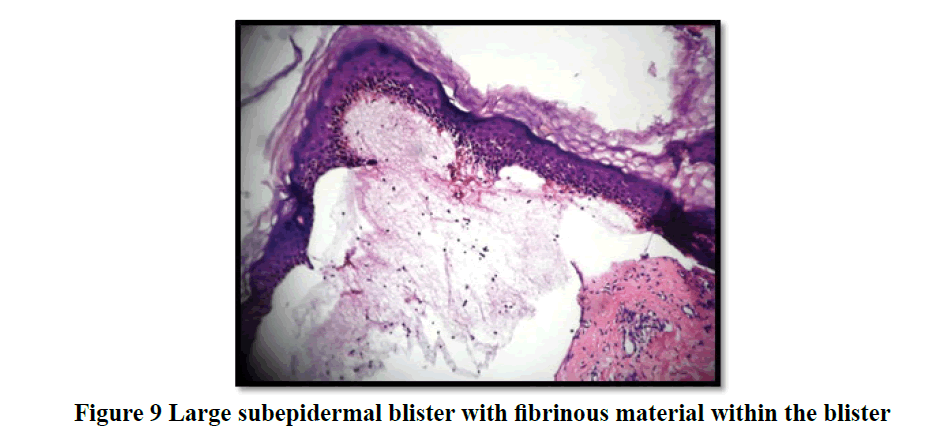
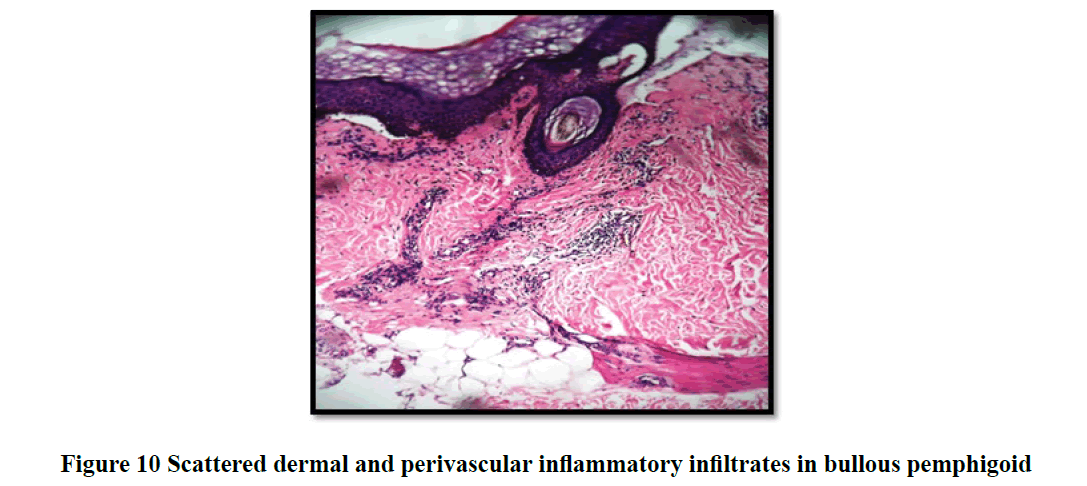
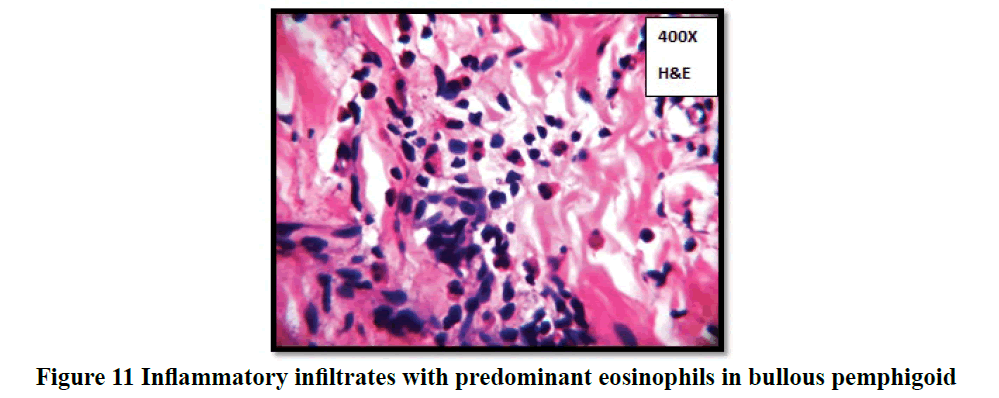
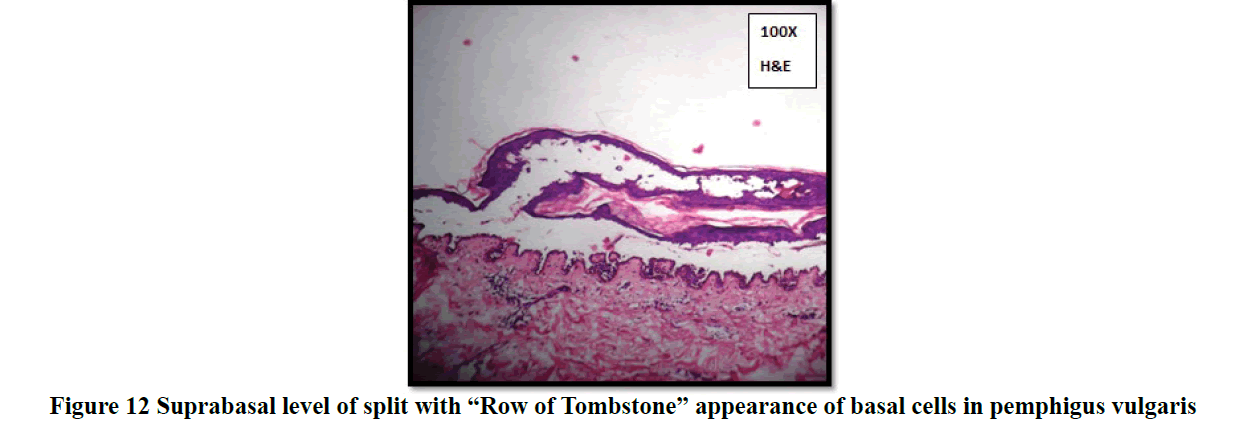
Histopathological evaluation showed 52.3% (58/111) of vesiculobullous diseases with subepidermal blister and 28.8% (32/111) with a suprabasal blister in the present study (Table 3). In 49/111 cases (44.1%), inflammatory cell infiltrates were the most common content of the blister with eosinophils (42 cases; 37.8%) being the predominant cell type. Acantholytic cells were seen in only in 18.9% (21/111) of cases. Wide range of epidermal changes were observed, most frequent finding being parakeratotic hyperkeratosis (47 cases: 42.3%) and acantholytic cells (39 cases; 35.1%) (Figure 8). Scattered dermal, perivascular, and adnexal inflammatory infiltrates were seen in almost all cases regardless of the level of blister formation. Epithelial regeneration (29.7%) was an interesting finding observed, that created a lot of dilemma regarding the level of split when biopsied from older lesions. Figures 9-17 show some of the microscopic pictures of the various vesiculobullous diseases seen in the present study. Direct Immunofluorescence (DIF) was done only in doubtful cases where histopathological findings were not confirmatory. In 36% (40/111) of cases, DIF was not attempted as the findings were straightforward and definite diagnosis could be obtained from histomorphology.
| Level of split | Frequency | Percent |
|---|---|---|
| Subepidermal | 58 | 52.3 |
| Suprabasal | 32 | 28.8 |
| Subcorneal | 9 | 8.1 |
| Intraepidermal | 9 | 8.1 |
| No blister | 3 | 2.7 |
Subepidermal blisters were seen in 52.3% (58/111) of cases and suprabasal blister were seen in 28.8% (32/111) of cases of vesiculobullous diseases.
Table 3: Distribution of the anatomical level of split in the study
Wide range of epidermal changes was observed in the study population. The most frequent finding noted along with blister was parakeratotic hyperkeratosis (42.3%) followed by acantholytic cells (35.1%), spongiosis (20.7%), acanthosis (13.5%), basal cell layer disruption (9.9%), crusting (9%) and papillomatosis (1.8%).
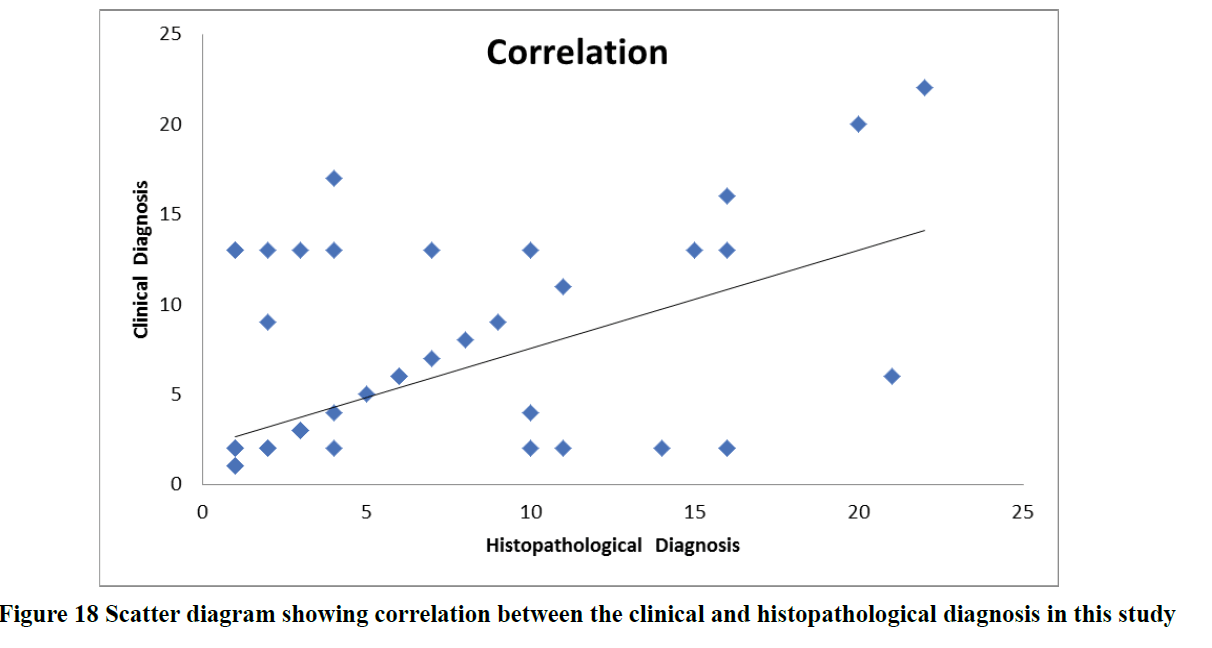
Based on histologic features, most of the autoimmune vesiculobullous diseases were found to be bullous pemphigoid (46 cases; 41%) followed by pemphigus vulgaris (29 cases; 26%) and pemphigus foliaceous (7 cases; 6%). The minor proportion of cases included Darier’s disease (4%), DH (2%), epidermolysis bullosa (2%) and 1% of SCPD, Hailey- Hailey disease, Drug Induced Pemphigus (DIP) , Lichen Planus Pemphigoid (LPP), erythema multiforme, atopic dermatitis, mucous membrane pemphigoid and CBDC each respectively (Table 4). Since pemphigus vulgaris and bullous pemphigoid were the most common blistering diseases seen in this study, their clinical and histopathological profile were studied in detail. The clinical characteristics and histomorphologic findings of pemphigus vulgaris (Table 5 and 6) and bullous pemphigoid (Table 7 and 8) in the study population are tabulated below. The clinical and histopathological concordance between various vesiculobullous diseases in the study is depicted in (Table 9). On analysis, we found that there was a positive Pearson Correlation of 0.546 between clinical and histopathological diagnosis (Table 10, Figure 18).
| Histopathologic diagnosis | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | Frequency | Percent | |
| Bullous pemphigoid | 12 | 32 | 34 | 47 | 46 | 41 |
| Pemphigus vulgaris | 9 | 23 | 20 | 27 | 29 | 26 |
| Repeat biopsy indicated | 8 | 21 | 4 | 6 | 12 | 11 |
| Pemphigus foliaceous | 2 | 5 | 5 | 7 | 7 | 6 |
| Darier’s disease | 2 | 5 | 3 | 4 | 5 | 4 |
| Dermatitis herpetiformis | 0 | 0 | 2 | 3 | 2 | 2 |
| Epidermolysis bullosa | 1 | 3 | 1 | 1 | 2 | 2 |
| Subcorneal pustular dermatosis | 0 | 0 | 1 | 1 | 1 | 1 |
| Hailey-Hailey disease | 1 | 3 | 0 | 0 | 1 | 1 |
| Drug induced | 1 | 3 | 0 | 0 | 1 | 1 |
| Lichen planus pemphigoid | 0 | 0 | 1 | 1 | 1 | 1 |
| Erythema multiforme | 0 | 0 | 1 | 1 | 1 | 1 |
| Atopic dermatitis | 0 | 0 | 1 | 1 | 1 | 1 |
| Mucous membrane pemphigoid | 1 | 3 | 0 | 0 | 1 | 1 |
| CBDC | 1 | 3 | 0 | 0 | 1 | 1 |
Chi square 0.194; p value >0.05.
Based on histological features, most of the autoimmune vesiculobullous diseases were found to be bullous pemphigoid (41%) followed by pemphigus vulgaris (26%) and pemphigus foliaceous (6%). Most of above-mentioned diseases were more frequent in females than in males.
Table 4: Distribution of histopathological diagnosis in the study
| Clinical characteristics | Frequency | Percent |
|---|---|---|
| Sex | ||
| Male | 9 | 31 |
| Female | 20 | 69 |
| Site of onset | ||
| Oral Lesion | 12 | 41.4 |
| Trunk | 3 | 10.3 |
| Extremities | 2 | 6.9 |
| Axilla and groin | 4 | 13.8 |
| Scalp and face | 5 | 17.2 |
| Generalised | 3 | 10.3 |
| Duration of disease | ||
| 1 week to 1 month | 6 | 20.7 |
| > 1 month to 1 year | 15 | 51.7 |
| ≥ 1 year | 8 | 27.6 |
| Symptoms | ||
| Vesicles and bullae | 14 | 48.3 |
| Vesicles and bullae+itching | 14 | 48.3 |
| Vesicles and bullae+itching+raised papules/plaques+pain | 1 | 3.4 |
| Mucous membrane lesion | 24 | 82.8 |
| Co-morbidities | 4 | 13.8 |
| Papule | 3 | 10.3 |
| Vesicles and bullae | 29 | 100 |
| Appearance | ||
| Flaccid | 9 | 31 |
| Tense | 9 | 31 |
| Both | 11 | 37.9 |
| Adjacent skin | 18 | 62.1 |
| Nikolsky sign | 14 | 48.3 |
| Bullae spread sign | 15 | 51.7 |
| Pustule | 3 | 10.3 |
| Erosions | 7 | 24.1 |
| Excoriations | 2 | 6.9 |
| Crust | 11 | 37.9 |
| Pigmentations | ||
| Absent | 26 | 89.7 |
| Hyperpigmented | 3 | 10.3 |
| Distribution of lesion | ||
| Intertriginous areas | 1 | 3.4 |
| Generalised | 22 | 75.9 |
| Head, neck, oral and trunk | 3 | 10.3 |
| Head, neck, trunk and extremities | 2 | 6.9 |
| Trunk and extremities | 1 | 3.4 |
Table 5: Clinical characteristics of pemphigus vulgaris
| Histological characteristics | Frequency | Percent |
|---|---|---|
| Tzanck smear | ||
| Acantholytic cells only | 8 | 27.6 |
| Inflammatory cells only | 1 | 3.4 |
| Acantholytic cells+inflammatory cells | 8 | 27.6 |
| Level of split | ||
| Intra epidermal | 4 | 13.8 |
| Suprabasal | 25 | 86.2 |
| Content of the blister | ||
| Acantholytic and dyskeratotic cells | 4 | 13.8 |
| Inflammatory cells | 7 | 24.1 |
| Fibrinous material and inflammatory cells | 1 | 3.4 |
| Acantholytic and dyskeratotic and inflammatory cells | 17 | 58.6 |
| Predominant cell | ||
| Neutrophils | 13 | 44.8 |
| Lymphocytes | 2 | 6.9 |
| Acantholytic cells | 14 | 48.3 |
| Hyperkeratosis/ Parakeratosis | 11 | 37.9 |
| Crusting | 2 | 6.9 |
| Keratotic plugging | 1 | 3.4 |
| Papillomatosis absent | 29 | 100 |
| Acanthosis | 1 | 3.4 |
| Spongiosis | 12 | 41.4 |
| Exocytosis | 2 | 6.9 |
| Dyskeratosis | 1 | 3.4 |
| Acantholytic cells | 25 | 86.2 |
| Necrotic keratinocytes | 1 | 3.4 |
| Basal cell layer disrupted | 1 | 3.4 |
| Dermal edema | 2 | 6.9 |
| Papillary microabscesses absent | 29 | 100 |
| Melanin incontinence | 8 | 27.6 |
| Epithelial regeneration | 12 | 41.4 |
| Dermal infiltrate | 22 | 75.9 |
| Perivascular infiltrate | 23 | 79.3 |
| Vasculitis absent | 29 | 100 |
| Involvement of follicular epithelium | 3 | 10.3 |
| Adnexal infiltrate | 13 | 44.8 |
| DIF indicated | 20 | 69 |
Table 6: Histopathological findings of pemphigus vulgaris
| Clinical characteristics | Frequency | Percent |
|---|---|---|
| Sex | ||
| Male | 12 | 26.1 |
| Female | 34 | 73.9 |
| Site of onset | ||
| Oral lesion | 4 | 8.7 |
| Trunk | 12 | 26.1 |
| Extremities | 8 | 17.4 |
| Axilla and groin | 3 | 6.5 |
| Scalp and face | 9 | 19.6 |
| Generalised | 10 | 21.7 |
| Duration of disease | ||
| 1 week to 1 month | 18 | 39.1 |
| >1 month to 1 year | 22 | 47.8 |
| ≥ 1 year | 6 | 13 |
| Symptoms | ||
| Itching | 1 | 2.2 |
| Vesicles and bullae | 10 | 21.7 |
| Both | 32 | 69.6 |
| Pigmented raised lesions | 2 | 4.3 |
| Vesicles and bullae+Itching+Raised papules/plaques+Pain | 1 | 2.2 |
| Mucous membrane lesion | 14 | 30.4 |
| Co-morbidities | 7 | 15.21 |
| Papule | 5 | 10.9 |
| Vesicles and bullae | 43 | 93.5 |
| Appearance | ||
| Flaccid | 6 | 13 |
| Tense | 19 | 41.3 |
| Both | 18 | 39.1 |
| Adjacent skin erythematous | 9 | 19.6 |
| Nikolsky sign | 5 | 10.9 |
| Bullae spread sign | 6 | 13 |
| Pustule | 0 | 0 |
| Erosions | 8 | 17.4 |
| Excoriations | 5 | 10.9 |
| Crust | 11 | 23.9 |
| Pigmentations-absent | 40 | 87 |
| Distribution of lesion | ||
| Head and neck | 1 | 2.2 |
| Trunk | 1 | 2.2 |
| Extremities | 4 | 8.7 |
| Intertriginous areas | 1 | 2.2 |
| Generalised | 23 | 50 |
| Head, neck, oral and trunk | 2 | 4.3 |
| Head, neck, trunk and extremities | 12 | 26.1 |
Table 7: Clinical characteristics of bullous pemphigoid
| Histological Characteristics | Frequency | Percent |
|---|---|---|
| Tzanck smear-inflammatory cells only | 13 | 28.3 |
| Level of split-subepidermal | 46 | 100 |
| Content of the blister | ||
| Fibrinous material | 2 | 4.3 |
| Inflammatory cells | 20 | 43.5 |
| Fibrinous material and inflammatory cells | 24 | 52.2 |
| Predominant cell | ||
| Eosinophils | 40 | 87 |
| Lymphocytes | 4 | 8.7 |
| Hyperkeratosis/parakeratosis | 15 | 32.6 |
| Crusting | 2 | 4.3 |
| Keratotic plugging | 1 | 2.2 |
| Papillomatosis absent | 46 | 100 |
| Acanthosis | 4 | 8.7 |
| Spongiosis | 1 | 2.2 |
| Exocytosis absent | 46 | 100 |
| Dyskeratosis absent | 46 | 100 |
| Acantholytic cells absent | 46 | 100 |
| Necrotic keratinocytes | 3 | 6.5 |
| Basal cell layer disrupted | 7 | 15.2 |
| Dermal edema | 4 | 8.7 |
| Papillary microabscess absent | 46 | 100 |
| Melanin incontinence | 15 | 32.6 |
| Epithelial regeneration | 14 | 30.4 |
| Dermal infiltrate | 36 | 78.3 |
| Perivascular infiltrate | 36 | 78.3 |
| Vasculitis absent | 46 | 100 |
| Involvement of follicular epithelium-absent | 46 | 100 |
| Adnexal infiltrate | 24 | 52.2 |
| DIF -indicated | 35 | 76.1 |
Table 8: Histopathological findings in bullous pemphigoid
| Histopathological | Pemphigus vulgaris | Bullous pemphigoid | Pemphigus foliaceous | Dermatitis herpetiformis | Subcorneal Pustular Dermatosis | Darier’s disease | Hailey-Hailey disease | Drug induced | Epidermolysis bullosa | Lichen planus pemphigoid | Repeat biopsy indicated | Erythema multiforme | Atopic dermatitis | Mucous membrane pemphigoid | CBDC | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical diagnosis | ||||||||||||||||
| Bullous lesion of lupus erythematosus | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| Bullous pemphigoid | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 34 |
| CBDC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Darier’s disease | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Dermatitis herpetiformis | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 4 |
| Drug induced | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Epidermodysplasia verruciformis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Epidermolysis bullosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Erythema multiforme | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 4 |
| Hailey-Hailey disease | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Lichen planus pemphigoid | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Linear IgA dermatosis | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mucous membrane pemphigoid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Paraneoplastic pemphigus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Pemphigus vulgaris | 29 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 42 |
| Pemphigus foliaceous | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 8 |
| Subcorneal pustular dermatosis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 29 | 46 | 7 | 2 | 1 | 5 | 1 | 1 | 2 | 1 | 12 | 1 | 1 | 1 | 1 | 111 |
Table 9: Clinical and histopathological concordance between various vesiculobullous diseases
| Clinical diagnosis | Histopathological diagnosis | ||
|---|---|---|---|
| Clinical diagnosis | Pearson correlation | 1 | .546** |
| Sig. (1-tailed) | 0 | ||
| N | 111 | 111 | |
| Histopathological diagnosis | Pearson correlation | .546** | 1 |
| Sig. (1-tailed) | 0 | ||
| N | 111 | 111 | |
**: Correlation is significant at the 0.01 level (1-tailed).
Table 10: Correlation between the clinical and histopathological diagnosis in this study
The above tabular column and scatter diagram shows a positive Pearson Correlation of 0.546 between clinical and histopathological diagnosis.
Discussion
Autoimmune vesiculobullous diseases are characterized by varying levels of blister formation, within or below the epidermis, based on the antigen being targeted. The early history, clinical and histological differentiation of various blistering diseases was beautifully described in a classic monograph by a pioneer in the field, Walter Lever in 1965 [6]. Pemphigus diseases were described way back in 360-450 BC by Hippocrates who described pemphigoid fever as “pemphigodes pyertoi.” Later in 1791 Wichmann coined the term “pemphigus” [7]. It was Lever who after analysing previous data’s and studying his own patients, clearly defined Bullous Pemphigoid in 1953 as a distinct disease [8]. Stanley et al. Labib et al. and Diaz et al in their studies demonstrated that autoantibodies were directed against adhesion structures in the skin and mucous membrane, a conclusion gratifying the pathology of these autoimmune blistering diseases [9].
Though various primary cutaneous diseases present clinically with vesiculobullous lesions, their aetiology, pathogenesis, severity, and course differ. Therefore, accurate diagnosis of these diseases is essential for appropriate management to avoid or minimize associated morbidity and mortality. Histopathological examination confirms the diagnosis in most cases of blistering diseases. It is a simple and cost-effective method of early diagnosis in places, where sophisticated techniques are not available. The classification of autoimmune vesiculobullous diseases is based on the microscopic evaluation of the blister which includes the level of the plane of separation, the type of cellular changes and the type of inflammatory cell infiltrates [10]. Immunofluorescence techniques are essential to supplement clinical findings and histopathologic features in the diagnosis of autoimmune vesiculobullous disorders, among them Direct Immunofluorescence (DIF) is the best method for the detection of immunocomplex deposition that can be seen in most autoimmune lesions especially in subepidermal blistering diseases [11,12].
The present study analysed in depth the histological patterns of various autoimmune blistering diseases and compared it with the clinical findings observed. One hundred and eleven histologically diagnosed cases of vesiculobullous diseases were studied during the two-year period, which is one of the largest in Indian literature and this constituted 5.28% of all skin biopsy specimens received during that period [13-15]. In the present study there was an overall female preponderance (65.8%) which was comparable to most of the western studies and a study conducted by Arundhathi et al., [16]. The higher incidence among females could be attributed to higher incidence of autoimmune conditions among females. Majority of cases were in the 5th to 7th decade of life with a mean age of 51.88 ± 32.16 which was similar to various Indian studies but was slightly different from the western studies where the mean age was higher. Vesicles and bullae (87%) were the most common clinical presentation with generalised distribution. Majority of the study population did not have co-morbidities (85%). Among vesiculobullous diseases, co-morbidities have been well studied only in BP. In a study done by Teixeira et al., diabetes mellitus was associated with bullous pemphigoid in 26%, but they could not demonstrate any significant association [17]. The proposed mechanism for the occurrence of diabetes mellitus among patients is that an autoimmune response occurs after exposure of the BP antigens, by glycation of proteins of the dermo-epidermal junction. In our study, only 8 patients had diabetes mellitus out of which 7 had bullous pemphigoid.
The clinical provisional diagnosis and histopathological diagnosis given are shown in Table 5. Majority of the studies in the past have analysed individual vesiculobullous diseases and only extremely limited studies have observed the histological findings of the entire study population. In the present study, subepidermal level of split (52.3%) was the most common finding. This could be attributed to the fact that bullous pemphigoid constituted the maximum number of cases histologically. This was followed by suprabasal level of split in 28.8% cases. Inflammatory cell infiltrates were seen in 44.1% of cases, out of which eosinophils constituted 37.8%, neutrophils 27.9% and lymphocytes 10.8%. Arundhathi et al., found that blisters were associated with epidermal changes [16]. In our study, 42.3% showed associated hyperkeratosis/parakeratosis, 35.1% showed acantholytic changes of adjacent epidermal cells with 64.10% of them seen in pemphigus vulgaris cases. Similarly, spongiosis was seen in 20.7% with 52.17% of them seen again in pemphigus vulgaris. This was comparable to the study done by Leena et al., on the changes noted in pemphigus vulgaris cases [15]. Acanthosis, disrupted basal cell layer, papillomatosis, dyskeratosis, necrotic keratinocytes and exocytosis were seen only in minor proportion. Significant numbers of dermal changes were also noted along with epidermal findings in most cases, majority of which were inflammatory type of reaction. Scattered dermal infiltrates and perivascular infiltrates were seen to go hand in hand in almost all cases. These findings were more common in subepidermal blistering disorders like bullous pemphigoid (42.85%) than in intraepidermal blisters like pemphigus vulgaris (26.19%). Acanthoytic change of follicular epithelium was an interesting finding observed in 5 cases (4.5%), all of which were seen in pemphigus vulgaris cases. This finding is important as it helps to differentiate pemphigus from other suprabasal blistering disease. 2 cases (1.8%) showed features of vasculitis in which one was diagnosed as erythema multiforme and the other as atopic dermatitis. Only 1 case (0.9%) showed papillary microabscesses which was diagnosed as dermatitis herpetiformis. Direct immunofluorescence was done only in cases where clinical features and histological findings were not contributory, thereby avoiding unnecessary financial burden and ensure early initiation of treatment.
Based on the above mentioned histomorphologic features and clinical history complemented by direct immunofluorescence studies wherever needed, a final diagnosis was arrived at. In the present study, bullous pemphigoid (41%), followed by pemphigus vulgaris (26%) were the most common autoimmune vesiculobullous diseases observed. This may be due to the increased incidence of autoimmune blistering disease as a whole along with increased number of patients, being a tertiary healthcare centre. The reason for the increased incidence of bullous pemphigoid could be due to the geographical distribution and the changing pattern of the disease. In 12 cases out of 111 cases (11%), the histological features were not contributory and hence were advised for repeat biopsy. The non-contributory cases was due to various factors like the site of the lesion where skin biopsy was performed, errors in fixation, improper handling of the delicate skin biopsy specimens during grossing, embedding and processing of slides where epidermis maybe lost at times if not sectioned properly. These however were not found to be significant in the present study.
In the study population, bullous pemphigoid was the most common vesiculobullous disorder encountered which was quite unique when compared to other Indian studies conducted. Western studies showed an increased incidence of bullous pemphigoid when compared to pemphigus vulgaris [14,16,18]. Thirty four cases (31%) were clinically diagnosed as bullous pemphigoid, out of which 32 cases were histologically confirmed to be the same, 1 turned out to be epidermolysis bullosa and a repeat biopsy was indicated in the other. Fourteen cases clinically diagnosed as various other blistering diseases were histologically confirmed to be bullous pemphigoid. Bullous pemphigoid cases in the present study showed a female (73.9%) predominance which was comparable to various other studies reviewed [13]. Wide ranges of age differences were noted among the 46 cases. Maximum numbers of cases were seen in the age group between (40-69) years (24 cases) in accordance with other studied in Indian literature. The youngest was 12 years of age and the oldest was 95 years of age. Western population showed a slightly higher range with maximum cases between (75-90) years. Oral lesion as the primary site was noted only in 8.7% of cases in contrast to pemphigus vulgaris cases where it constituted to 41.4% cases. Mucous membrane involvement was noted in only 14 cases (30.4%) when compared to pemphigus vulgaris cases (82.8%) which had more mucous membrane involvement in par with most studies observed. In 19 cases (41.3%) the vesicles were tense and were seen arising from an erythematous skin (Figure 3) like in study conducted by Leena et al [15]. Seven cases (15.21%) were associated with co-morbidities like diabetes mellitus, hypertension, or both. This is comparable to the results of the study done by Teixeira et al where diabetes mellitus was present in 26%. All cases of histologically diagnosed bullous pemphigoid showed subepidermal blister (100%) with 24 out of 46 cases (52.2%) showing fibrinous material along with inflammatory cells as the content of the blister as shown in Figure 9. Eosinophils formed the most predominant inflammatory cell type constituting 87% cases [19]. Spongiosis, acantholysis and crusting were less frequent when compared to pemphigus vulgaris cases, where these were the predominant changes associated. Epithelial regeneration was noted in 30.4% cases (14 cases) and could be due to the varying ages of blister formation in these cases. In 35 cases (76.1%) of bullous pemphigoid, DIF was advised/done, as histomorphological findings were not confirmatory. This could be explained by the vast majority of differential diagnosis for subepidermal blistering diseases which have a common clinical manifestation and hence requires the use of immunofluorescence for confirmation by the detection of specific immune complex deposition.
The present study was done to characterize different histological findings in detail and to compare it with the clinical diagnosis of blistering diseases. They were then statistically correlated using Pearson correlation and found to have a good positive correlation of 0.546. A positive score indicates that there is a good correlation between the clinical diagnosis and final histological diagnosis in most cases as in our study. This emphasizes the need for histopathological study in vesiculobullous diseases to arrive at a definite diagnosis which would benefit the patient both from prognostic and treatment point of view.
Pemphigus vulgaris was clinically diagnosed in 42 cases (38%), out of which histopathological diagnosis correlated in 29 patients (69%). In 8 cases (17.4%), pemphigus vulgaris was later histologically diagnosed to be bullous pemphigoid. This could be due to the heterogeneous clinical manifestations. In 5 cases (11.9%), the report was signed out as intra-epidermal blistering disease and repeat biopsy was advised as the characteristic histological findings of pemphigus were not seen. This may be due to skin biopsies taken from old blisters or cases associated with papules, excoriations, pustules or crusts or due epithelial regeneration or associated epidermal findings like crusting or erosions which makes the histopathological diagnosis difficult. Bullous pemphigoid was clinically diagnosed in 34 cases (31%), out of which 32 cases (69.6%) had histomorphologic correlation. One case (2.9%) was diagnosed as epidermolysis bullosa and in the other, a repeat biopsy was advised. Bullous pemphigoid cases did not cause much difficulty in arriving at a histological diagnosis. Out of 8 cases (7%) clinically diagnosed as pemphigus foliaceous, 7 cases (87.5%) had histomorphologic correlation. There was a very good clinical and histological correlation in pemphigus foliaceous cases. The histopathological changes noted were consistent, as compared to various other studies reviewed. All cases of Darier’s disease, CBDC, mucous membrane pemphigoid, epidermolysis bullosa, PNP, drug induced pemphigus and SCPD were clinically and histologically correlating (100%).
Conclusion
Vesiculobullous diseases cause significant morbidity and mortality to the patient if not detected and treated as soon as possible. In this study, bullous pemphigoid constituted the most common type of autoimmune vesiculobullous disease in this study followed by pemphigus vulgaris, which was quite unique when compared to other studies in Indian literature. This is probably due to the changing pattern of the disease especially in our population. Histopathological diagnosis correlated well with clinical diagnosis in most of the vesiculobullous disease, emphasizing the need for skin biopsy in all cases of vesiculobullous diseases. It is a cost effective out-patient procedure. Direct immunofluorescence is helpful in scenarios where clinical and/ or histopathological features are inconclusive. Considering the socioeconomic conditions of the patients and the unavailability of immunofluorescence technique widely, clinical diagnosis and histopathology forms the cornerstone in arriving at the diagnosis. DIF is only a supplement but not a substitute. This study helped in understanding the characteristic histopathological features of commonly encountered as well as the rare vesiculobullous disorders. These skin diseases are still life-threatening dermatological conditions that require constant efforts to elucidate the pathogenetic mechanisms behind the diseases and to develop new therapies that are needed to improve quality care for our patients. A future study with larger number of patients utilizing histological and DIF techniques could provide further insights into this group of often debilitating and even fatal diseases.
Declerations
Acknowlegement
The authors thank the faculties in the Department of Dermatology, Government Medical College, Trivandrum for their valuable contribution and input to complete the clinical proforma and follow-up of the patients included in this study.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Patterson, J. W. "Weedon's skin pathology." 4th ed. 2010, pp. 123-68.
- Collier, P. M., and F. Wojnarowska. "Blistering diseases." Medicine-Abingdon-UK Edition 25, 1997, pp. 51-5.
- Burns, Tony, et al., eds. "Rook's textbook of dermatology." John Wiley & Sons, 2008.
- Wu, H., B. Schapiro, and T. J. Harrist. "Noninfectious vesiculobullous and vesiculopustular diseases. Histopathology of the skin. Ed. Elder D, Elenitsas R, Jhonson Jr BL, Murphy GF. 9’uncu bask›." 2005, pp. 243-91.
- Alghanmi, Najla M., and Layla S. Abdullah. "Pathology of skin diseases." Saudi Medical Journal, Vol. 34, No. 1, 2013, pp. 74-9.
- Lever W.F. "Pemphigus and pemphigoid." Charles C. Thomas, Springfield, IL1965.
- Holubar, Karl. "Pemphigus: a disease of man and animal: historical and other perspectives." International Journal of Dermatology, Vol. 27, No. 7, 1988, pp. 516-20.
- Lever, Walter F. "Pemphigus." Medicine, Vol. 32, No. 1, 1953, pp. 1-123.
- Amagai, Masayuki, Vera Klaus-Kovtun, and John R. Stanley. "Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion." Cell, Vol. 67, No. 5, 1991, pp. 869-77.
- Rosai, Juan, and S. Ackerman. "Surgical pathology." Eigth edition, Mosby, 1996.
- Huilgol, S. C., B. S. Bhogal, and M. M. Black. "Immunofluorescence of the immunobullous disorders part one: Methodology." Indian Journal of Dermatology, Venereology and Leprology, Vol. 61, No. 4, 1995, pp. 187-95.
- Tariq, M., and S. H. Tahir. "Patterns of autoimmune bullous diseases of skin in Lahore." Pakistan. Journal of Pakistan Association of Dermatologists, Vol. 13, 2003, pp. 67-9.
- Bhalara, Rohit, et al. "A histopathological study of vesiculobullous lesions of skin: A study at PDU Medical College, Rajkot." International Journal of Research in Medicine, Vol. 2, No. 4, 2013, pp. 7-9.
- Kabir, AKM Nurul, Mohammed Kamal, and Aga Masood Choudhury. "Clinicopathological correlation of blistering diseases of skin." Bangladesh Medical Research Council Bulletin, Vol. 34, No. 2, 2008, pp. 48-53.
- Leena, J. B., et al. "A clinicopathological study of immunobullous lesions of the skin." Advance Laboratory Medicine International, Vol. 2, No. 2, 2012, pp. 49-60.
- Arundhathi, S., S. Ragunatha, and K. C. Mahadeva. "A cross-sectional study of clinical, histopathological and direct immunofluorescence spectrum of vesiculobullous disorders." Journal of Clinical and Diagnostic Research: JCDR, Vol. 7, No. 12, 2013, pp. 2788-92.
- Teixeira, Vera Barreto, et al. "Bullous pemphigoid and comorbidities: a case-control study in Portuguese patients." Anais Brasileiros de Dermatologia, Vol. 89, No. 2, 2014, pp. 274-8.
- Chan, P. T. "Clinical features and diagnosis of common autoimmune bullous disorder in Hongkong." Hong Kong Medical Diary, Vol. 13, 2008, pp. 12-5.
- Arun, T. The clinical and pathological study of autoimmune vesiculobullous disorders. Diss. 2011.