Research - International Journal of Medical Research & Health Sciences ( 2022) Volume 11, Issue 12
Comparison of the use of Carvedilol Versus Nebivolol in the Treatment of Acute Decompensation in the Patient with Chronic Heart Failure: Evaluation of Clinical variables
Jorge L. Z. Garcia, Francisco J. Sanchez, Gabriela Guanay and Maite Gonzalez and Jose H. Donis*Maite Gonzalez and Jose H. Donis, Institute of Cardiovascular Research, University of the Andes, Merida, Venezuela, Email: josedonis1945@gmail.com, donis_jose@hotmail.com
Received: 21-Nov-2022, Manuscript No. ijmrhs-22-80593; Editor assigned: 22-Nov-2022, Pre QC No. ijmrhs-22-80593 (PQ); Reviewed: 22-Nov-2022, QC No. ijmrhs-22-80593 (Q); Revised: 28-Nov-2022, Manuscript No. ijmrhs-22-80593 (R); Published: 20-Dec-2022
Abstract
Previous trials demonstrated the safety of using beta-blockers in the acute decompensation of heart failure; it is unknown whether the use of nebivolol translates into an improvement in clinical parameters at 96 hours, compared to the use of carvedilol in a similar study group. Objectives: To compare the effect of 2 treatment strategies, where the difference is made by the type of beta-blocker, in patients with acutely decompensated chronic heart failure. Methods: A single-center, prospective, experimental, randomized, double-blind clinical trial was carried out, 22 patients with LVEF ≤ 40% were randomly assigned to receive carvedilol or nebivolol with daily dose increase, and clinical variables were measured for 96 hours. Results: The carvedilol group reached a maximum dose of 33.3 ± 10 mg and nebivolol 9.37 ± 1.25 mg, with both treatment strategies compensation was achieved in more than 50% of the patients in both groups, without statistically significant differences for the majority of patients. clinical variables, except for greater weight loss in the carvedilol group, reaching an absolute reduction of 5.62 kg (95% CI 3.22 Kg-8.02 kg) versus nebivolol with 2.54 kg (95% CI 0. 14 kg-4.94 kg) at 96 hours of follow-up (p 0.001) Conclusions: In patients with acutely decompensated chronic heart failure and reduced Left Ventricular Ejection Fraction (LVEF), the use of beta-blockers is safe and well tolerated, guarantees clinical improvement and rapid compensation, with doses diuretic drops. The group with carvedilol showed greater weight reduction, compared to the nebivolol group in the study population
Keywords
Beta-blockers, Diuretics, Acutely decompensated Heart Failure
Introduction
The prevalence of Heart Failure (HF) is 1%-2% of the adult population in developed countries and increases to more than 10% among people older than 70 years of age, in turn, acute heart failure syndrome is defined as the appearance or recurrence of an episode of gradual or rapid deterioration of signs and symptoms, requiring urgent or emergent treatment, leading to hospital admission [1-3]. In the US alone, approximately 3 million patients are admitted each year with a diagnosis of primary or secondary IC [4]. It is known that the goals of treatment for patients with HF consist of improving clinical status, functional capacity, and quality of life, preventing hospitalizations, and reducing mortality. One of the fundamental pillars in the treatment of this pathology is beta-blockers, which in large clinical trials have shown to reduce morbidity and mortality in symptomatic patients with heart failure with reduced ejection fraction (HF-rFEVI) [5]. To date, it is stated that beta-blockers are better tolerated when the patient is less congestive, "dry" or euvolemic, with an adequate resting heart rate, and it is also suggested that they should not be started in patients with signs or symptoms of decompensation [6]. On the other hand, treatment with beta-blockers can be started in clinically stable patients at low doses, and then gradually increased until the maximum tolerated dose is reached [5]. In patients with LVEF <25%, NYHA functional class IV, a 31% reduction in all-cause mortality was observed [7]. In turn, nebivolol in patients older than 70 years, with a confirmed diagnosis of HF and higher LVEF or equal to 35% showed a 14% reduction in the combined variable of all-cause mortality and cardiovascular hospitalization [8]. In acutely decompensated patients, in whom carvedilol was started in the acute phase, a correlation was observed between the increase in heart rate and the degree of neurohormonal activation with parameters of left ventricular diastolic dysfunction, and in turn, they experienced a lower rate of events. during follow-up. The authors conclude that patients treated with ACE inhibitors, beta-blockers, and low-dose diuretics have better results in terms of neurohormonal activation and left ventricular filling dynamics. They also suggest that higher doses of diuretics are associated with a worse outcome and greater risk of events [9]. Over time it has been shown that the symptoms in patients with heart failure are predictors of poor prognosis. Fatigue and dyspnea, common symptoms in HF, have important and independent long-term prognostic implications [10]. High resting Heart Rate (HR) is associated with long-term adverse events [11]. A previous study evaluating changes in HR during hospitalization and the association between HR at discharge and clinical outcomes, as well as the interaction of beta-blocker therapy in patients with acute decompensated HF, showed that despite treatment with beta-blockers, many hospitalized patients with r-LVEF maintain levels relatively high HR at discharge and this was associated with higher mortality [12]. the magnitude of the reduction in heart rate is associated in a statistically significant way with the benefit in survival, while the dose of beta blockers does not have such a relationship [13]. In this sense, beta-blockers can reduce mortality by blocking adrenergic receptors and their relationship with the reduction of heart rate. Achieving a target heart rate range is an appropriate therapeutic goal for patients with HF [14].
Methods
A single-center, prospective, experimental, randomized, and double-blind clinical trial was carried out at the Institute of Cardiovascular Research of IAHULA Merida-Venezuela, between January 2021 and August 2021. Patients admitted to the cardiovascular acute care unit, between 18 years and 80 years of age, with a diagnosis of the clinical syndrome of acutely decompensated HF Clinical profile B according to the Stevenson Classification, in NYHA functional class III-IV, Ventricular Function (LVEF) less than 40%, who were not using beta-blockers at the time of income.
From admission to 96 hours of follow-up, the following was performed serially: measurement of systolic and diastolic blood pressure, oxygen saturation, daily fasting weight, and hourly diuresis plus fluid balance, as well as a standard 12-lead electrocardiogram; After the evaluation, those patients who met the inclusion criteria were assigned to receive the treatment for each protocol, without undergoing a washout period. Each treatment strategy was constituted as follows:
• Protocol 1: Enalapril 2.5 mg PO BID, Furosemide 20 mg IV OD, Digoxin 0.125 mg PO OD, Spironolactone 25 mg PO OD, Carvedilol, starting with 3.125 mg PO BID with progressive titration of dose according to clinical response. Maximum target dose 25 mg PO BID.
• Protocol 2: Enalapril 2.5 mg PO BID. Furosemide 20 mg EV OD. Digoxin 0.125 mg PO OD, Spironolactone 25 mg PO OD, and Nebivolol starting with 1.25 mg PO BID with progressive dose titration according to clinical response. Maximum target dose 5 mg PO BID.
The titration of the beta-blockers was carried out by tablet quarters. The investigator was blinded as to the type of beta-blocker the patient received, daily doubling of one or both doses was performed according to the patient's tolerance, until the maximum dose was reached, guaranteeing Systolic Blood Pressure (SBP) >90 mmHg, and monitoring the occurrence of adverse effects such as orthostatic hypotension, dizziness, bradycardia ≤ 50 bpm and others related to the use of beta-blockers. When there were criteria for anticoagulation, warfarin was added with progressive adjustment of the dose until reaching INR between 2 and 3. In cases that had criteria for antiplatelet therapy, acetylsalicylic acid was added at a dose of 81 mg PO OD in monotherapy or associated with clopidogrel 75 mg PO OD depending on the needs of the patient and following the guidelines of the international clinical practice guidelines. This protocol was approved by the research commission of the Cardiovascular Research Institute, and the informed consent of each patient was obtained.
Statistic Analysis
The Shapiro-Wilk test was performed to determine the normal distribution or not of the sample. The continuous numerical quantitative variables were expressed with measures of central tendency (means), and standard deviation, the categorical variables in terms of frequencies and percentages. Comparisons of continuous numerical variables between patients from protocols 1 and 2 were made using corresponding parametric or non-parametric tests and categorical variables with chi-square tests. For the comparison of intragroup and intergroup variables, T-Test and paired T-Test analyzes were performed. A value of p 0.05 was considered statistically significant. The values of the different data variables were collected by the researcher, and processed by a statistical analyst blind to the clinical information, with the IBM SPSS Statistics Software Version 20.0.
Results
A total of 22 patients met the inclusion criteria and were entered into the study randomly, 10 patients for the carvedilol group and 12 patients for the nebivolol group
The follow-up time was 96 hours. Table 1 shows the baseline characteristics of the studied population. There were no statistically significant differences between groups, the mean age for the carvedilol group was 61.8 ± 7.8 years and for the nebivolol group 68.2 ± 10.6 years. It should be noted that the majority of patients included in the study were men. 63% of the patients had a history of hypertension, 23% with a history of ischemic heart disease and atrial fibrillation, 24% with a history of diabetes mellitus, and 14% had marked alcoholic habits.
| Characteristics | Carvedilol | Nebivolol | p-value |
|---|---|---|---|
| (n=10) | (n=12) | ||
| Demographic variables | |||
| Age (years) | 61.8 ± 7.8 | 68.2 ± 10.6 | 0.128 |
| Sex (M/F) | 10/0 | 08/4 | 0.068 |
| Background (%/n) | |||
| Arterial hypertension | 57/8 | 45/6 | NS |
| Ischemic heart disease | 40/2 | 60/3 | NS |
| DM II | 50/3 | 50/3 | NS |
| atrial fibrillation | 60/3 | 40/3 | NS |
| Alcoholic habits | 67/3 | 33/1 | NS |
| Clinical variables | |||
| Weight (kg) | 77.2 ± 13.3 | 71.2 ± 16.1 | 0.36 |
| Heart rate (bpm) | 106.8 ± 18.8 | 103.7 ± 26.1 | 0.76 |
| Respiratory rate (rpm) | 24.3 ± 3 | 27.9 ± 6.1 | 0.11 |
| Systolic Pressure (mmHg) | 131 ± 27.6 | 132 ± 23.7 | 0.89 |
| Diastolic Pressure (mmHg) | 81 ± 14.4 | 82.5 ± 10.5 | 0.78 |
| Functional class (III/IV) | 04/06 | 05/07 | 0.63 |
| Edema M.I. (I/II/III/IV) | 0/1/1/8 | 1/4/3/4 | 0.17 |
| Crackles (yes/no) | 10/0 | 12/0 | NS |
| 3rd sound (Yes/No) | 04/06 | 04/08 | 0.31 |
| Electrocardiographic variable | |||
| Rhythm (sinus/AF) | 07/03 | 10/02 | 0.4 |
| PR interval (sec) | 0.17 ± 0.04 | 0.15 ± 0.01 | 0.27 |
| QRS complex (sec) | 0.09 ± 0.01 | 0.10 ± 0.02 | 0.3 |
| QT interval (sec) | 0.33 ± 0.03 | 0.31 ± 0.08 | 0.54 |
| Qtc interval (sec) | 0.41 ± 0.05 | 0.42 ± 0.05 | 0.9 |
| Paraclinical variables | |||
| Hemoglobin (Gr/dl) | 12.6 ± 2.01 | 13.3 ± 1.9 | 0.39 |
| Hematocrit (%) | 40.4 ± 8.8 | 39.5 ± 7.6 | 0.85 |
| Leukocytes (u/ml) | 7380 ± 1589 | 7868 ± 2814 | 0.63 |
| Glycemia (mg/dl) | 114.4 ± 38 | 138.7 ± 81.9 | 0.55 |
| Urea (mg/dl) | 54.05 ± 26.4 | 52.8 ± 27.2 | 0.92 |
| Creatinine (mg/dl) | 1.3 ± 0.4 | 1.1 ± 0.2 | 0.4 |
| Echocardiographic variables | |||
| LVAD (mm) | 59.7 ± 4.5 | 56.1 ± 6.5 | 0.16 |
| Mass index (gr/m2/sc) | 127.3 ± 20.6 | 144.7 ± 29.7 | 0.11 |
| LVEF (%) | 20.7 ± 5.7 | 25.5 ± 7.4 | 0.13 |
| Diastolic function (I/II/III) | 1/2/4 | 0/4/6 | 0.45 |
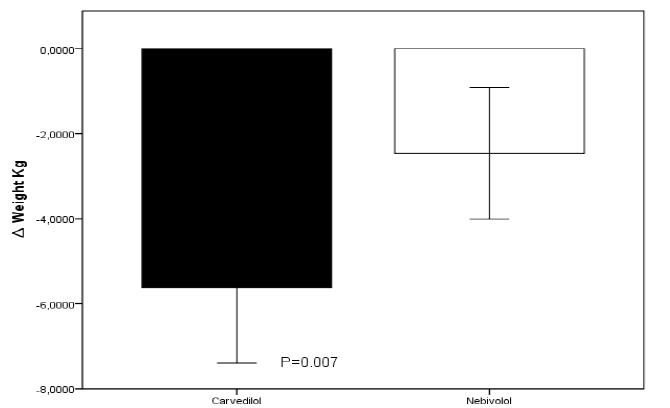
On admission, the average weight was 77.2 ± 13.3 kg for the carvedilol group and 71.2 ± 16.1 kg for the nebivolol group (p=0.36), the average heart rate was 106.8 ± 18.8 bpm and 103.7 ± 26.1 bpm for the carvedilol and nebivolol group respectively. Most of the patients in both groups were in NYHA classes III and IV, had signs of congestion, and were in sinus rhythm. The mean left ventricular ejection fraction was 20.7 ± 5.7% for the carvedilol group and 25.5 ± 7.4% for the nebivolol group (p=0.13). The average maximum tolerated dose of carvedilol was 33.3 ± 10 mg and for nebivolol 9.37 ± 1.25 mg at 96 hours of follow-up, without recording adverse events attributable to the use of beta-blockers; on the other hand, protocol 1 group received an average dose of furosemide of 20 mg while protocol 2 group received an average of 25 ± 9 mg with no statistically significant difference between groups (p 0.09), this because 3 patients of this group required an emergency dose of the diuretic. The clinical characteristics of both groups at 96 hours of follow-up are presented in Table 2. Clinical improvement was observed with both treatment strategies with no statistically significant difference between the two groups for most of the variables studied. Daily body weight decreased from the first 24 h to 96 h post-treatment, with both therapeutic strategies. There were no significant changes between groups during the first 72 hours, but there was an overall statistically significant difference, at the end of 96 hours of follow-up, in favor of the carvedilol group, reaching an average absolute reduction of 5.62 kg (95% CI). 3.22-8.02 p 0.007) versus the group treated with nebivolol in whom a mean weight loss of 2.54 kg (95% CI 0.14-4.94 p 0.007) was recorded at 96 hours after follow-up with a statistically significant difference between both groups p 0.001 (Figure 1)
| Characteristics | Carvedilol | Nebivolol | p-value |
|---|---|---|---|
| (n=10) | (n=12) | ||
| Clinical Variables | |||
| Weight (kg) | 71.6 ± 13.8 | 68.8 ± 17.3 | 0.68 |
| â?? Total weight (kg) | -5.62 ± 2.4 | -2.54 ± 2.4 | 0.007 |
| Heart rate (bpm) | 76.4 ± 10 | 69.6 ± 13.3 | 0.2 |
| â?? Total HR (bpm) | -30.4 ± 15.4 | -34.08 ± 18.4 | 0.62 |
| Respiratory rate (rpm) | 18.3 ± 1.5 | 19.3 ± 4.0 | 0.44 |
| Systolic pressure (mmHg) | 118 ± 23.4 | 119.5 ± 23.2 | 0.88 |
| â?? Total Pas (mmHg) | -13 ± 18.2 | -13 ± 25.5 | 0.9 |
| Diastolic pressure (mmHg) | 70.0 ± 15.6 | 76.2 ± 12.9 | 0.31 |
| â?? DBP (mmHg) | -11± 12.8 | -6.25 ± 12.2 | 0.38 |
| Functional class (II/III) | 07/03 | 06/06 | 0.3 |
| Edema mi (I/II/III/) | 03-03-2004 | 07-01-2004 | 0.58 |
| Crackles (yes/no) | 03/07 | 04/08 | 0.61 |
| Electrocardiographic variable | |||
| Rhythm (sinus/AF) | 07/03 | 10/02 | 0.4 |
| PR interval (sec) | 0.18 ± 0.04 | 0.16 ± 0.03 | 0.24 |
| QRS complex (sec) | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.91 |
| QT interval (sec) | 0.37 ± 0.05 | 0.40 ± 0.06 | 0.37 |
| Qtc interval (sec) | 0.42 ± 0.03 | 0.42 ± 0.05 | 0.82 |
| Treatment | |||
| Beta-blocker maximum dose reached (mg) | 33.3 ± 10 | 9.37 ± 1.25 | NS |
| Furosemide (mg/day) | 20 | 25 ± 9 | 0.09 |
| Enalapril (mg/day) | 5 | 5 | NS |
| Spironolactone (mg/day) | 25 | 25 | NS |
| Digoxin (mg/day) | 0.125 | 0.125 | NS |
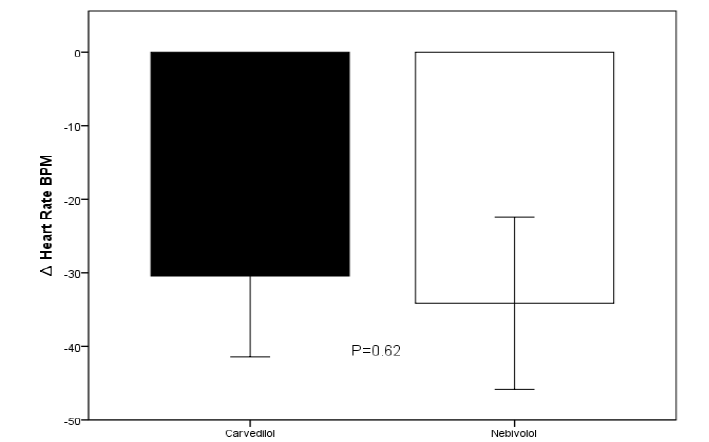
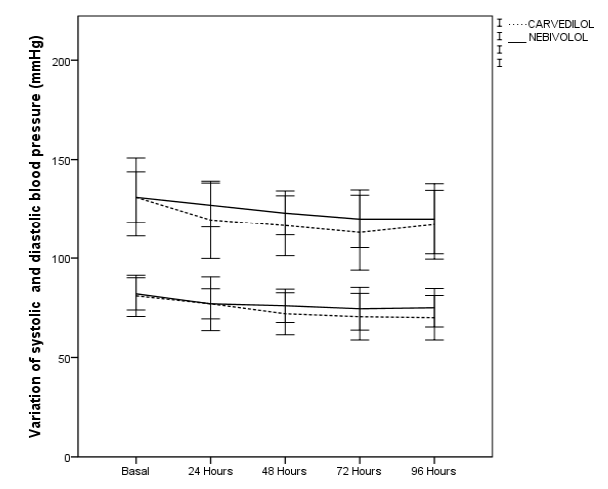
Most patients achieved NYHA functional class improvement, with 70% of patients achieving functional class II in the carvedilol group and 50% in the nebivolol group. The total decrease in mean heart rate was -30.4 ± 15.4 bpm for the carvedilol group and -34.08 ± 18.4 bpm for the nebivolol group, no relevant changes were recorded in electrocardiographic parameters such as prolongation of the P-R segment, QRS, QT interval, QTc (Figure 2). Aslight decrease in blood pressure figures was recorded without hemodynamic alterations orepisodes of hypotension during the 96 hours of follow-up (Figure 3)
Discussion
The purpose of this investigation was to compare the effect of medical treatment strategies, where the difference is made by the type of beta-blocker used, in patients with heart failure with acutely decompensated. Our results show that the clinical compensation given by a decrease in heart rate, respiratory rate, degree of jugular venous distention, crackles, and edema in the lower limbs, as well as improvement in functional class according to NYHA, was achieved with both strategies, but we also documented a greater decrease. in body weight at 96 hour follow-up in the carvedilol plus low-dose furosemide (20 mg/day) group. This finding could be explained by its nonselectivity properties, since this drug blocks α1 receptors and increases renal blood flow, improving glomerular filtration rate [15]. Renal sympathetic system activity is part of the final common pathway that leads to an increase in renal sodium reabsorption at the tubular level, and renal blood flow, both mediated by α1B and α1A adrenoceptors [16]. It allows us to understand that carvedilol improves diuresis, achieving greater decongestion, with the consequent loss of weight and improvement in functional class. The results of this investigation are consistent with previously published findings, conducted at our institution, where it was shown that the administration of the betablocker carvedilol during acute decompensation of systolic HF decreases sodium levels and urine osmolarity while generating urinary volumes similar to those induced by high doses of diuretics, suggesting that this beta-blocker exerts its compensatory action through renal excretion of free water [17]. The strategy of upward titration of carvedilol improved symptomatic status, functional class, neurohormonal activation, and left ventricular systolic function parameters in patients with newly diagnosed heart failure, in NYHA functional class II and III, including this strategy, allowed a significant reduction in the dose of diuretics during follow-up [18].
Regarding the use of nebivolol, a significantly lower weight loss was recorded in this group at 96 hours, in addition to the fact that in 3 of these patients it was necessary to administer an additional dose of emergency furosemide, which allows us to infer that at least in the analysis of this clinical variable, carvedilol was superior. The benefits of achieving greater weight loss would be translated into clinical benefits, such as those found in a previous investigation where weight loss, as well as fluid loss, and the reduction of NT-pro BNP at 72 hours are poorly correlated. with relief of dyspnea, however, changes in each of the 3 markers of decongestion were associated with a significant improvement in time to death, rate of readmissions, or 60-day emergency department visit, It is known that increased severity of congestion at discharge is associated with increased shortterm morbidity and mortality [19,20].
In contrast to a study carried out with 433 patients that aimed to determine the relationship between weight change during hospitalization and subsequent clinical events in patients with decompensated HF, they found an average weight loss of 3.6 kg during the first 96 hours of hospitalization, however, they did not observe significant differences between weight change and any in-hospital event or during follow-up, so these data challenge the merit of using weight as a surrogate endpoint for major clinical events such as hospitalization or death [21].
Both molecules achieved a significant reduction in heart rate, and these findings are relevant since it is known that maintaining high heart rates at discharge has been associated with greater mortality while the magnitude of the reduction in heart rate during hospitalization was associated with greater survival, for which reason reaching a target heart rate range is an ideal therapeutic objective for patients with acutely decompensated HF [14]. Short-term treatment with carvedilol at doses that induce comparable heart rate reductions has superior hemodynamic and metabolic effects compared with selective beta-blockers such as metoprolol CR/XL. These data suggest important advantages of blocking all three adrenergic receptor subtypes [22]. In this study, we observed that both beta-blockers, carvedilol, and nebivolol, managed to compensate the patients in this study group, mainly translated into an improvement in the NYHA functional class and a decrease in signs of congestion, which raises possible important clinical benefits attributable to these drugs. in the acute context; for example, greater in-hospital survival, as suggested by a Japanese study that aimed to evaluate the effect of the use of beta-blockers on admission on hospital mortality in 3,817 patients with acute decompensated heart failure, where they demonstrated that the use of beta-blockers on admission was significantly associated with a lower risk of cardiovascular mortality and a lower risk of non-cardiovascular mortality. (4.4% vs 7.6%, p<0.001). (odds ratio, 0.41; 95% CI, 0.27-0.60, p<0.001), in addition, the association of beta-blocker use with a lower risk of hospital mortality was relatively greater in patients receiving high doses of beta-blockers [23]. These findings are consistent with small studies, where the findings are similar, suggesting that progressive and cautious carvedilol titration, in patients still decompensated with sinus rhythm, increases long-term survival [24].
Limitations
We recognize limitations in terms of sample size, explained by a prospective study design dependent on the incidence of this clinical entity in our environment and the low influx of patients to our emergency room, possibly explained by the fear of the population. in the context of the COVID-19 pandemic. In addition, various pre-specified exclusion criteria were considered that limited the inclusion of a large number of patients in the study. This research was also limited by a greater predominance of male patients, we do not know if the findings found would have similar behavior in a more gender-balanced study group
Conclusion
The results of this study demonstrate the clinical benefit of the use of beta-blockers in the context of heart failure with acutely decompensated. Its use is safe and well tolerated, without significant adverse effects attributable to these drugs, which allows improvement of clinical variables and rapid compensation at 96 hours, with low doses of diuretics. The results about weight loss were favorable with the use of carvedilol compared to nebivolol, with no other relevant differences between the two beta-blockers. More research will be needed to confirm these findings.
Declarations
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
References
- Mosterd, Arend, and Arno W. Hoes. "Clinical epidemiology of heart failure." Heart, Vol. 93, No. 9, 2007, pp. 1137-46.
Google Scholar Crossref - Redfield, Margaret M., et al. "Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic." Jama, Vol. 289, No. 2, 2003, pp. 194-02.
Google Scholar Crossref - Mann, Douglas L., and Michael R. Bristow. "Mechanisms and models in heart failure: the biomechanical model and beyond." Circulation, Vol. 111, No. 21, 2005, pp. 2837-49.
Google Scholar Crossref - Gheorghiade, Mihai, and Peter S. Pang. "Acute heart failure syndromes." Journal of the American College of Cardiology, Vol. 53, No. 7, 2009, pp. 557-73.
Google Scholar - Tsutsui, Hiroyuki, et al. "JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure-digest version." Circulation Journal, Vol. 83, No. 10, 2019, pp. 2084-84.
Google Scholar Crossref - Maddox, Thomas M., et al. "2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee." Journal of the American College of Cardiology, Vol. 77, No. 6, 2021, pp. 772-10.
Google Scholar - Packer, Milton, et al. "Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study." Circulation, Vol. 106, No. 17, 2002, pp. 2194-99.
Google Scholar Crossref - Flather, Marcus D., et al. "Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS)." European heart journal, Vol. 26, No. 3, 2005, pp. 215-25.
Google Scholar Crossref - Reina-Couto, Marta, et al. "Endothelitis profile in acute heart failure and cardiogenic shock patients: Endocan as a potential novel biomarker and putative therapeutic target." Frontiers in physiology, 2022. P. 1517.
Google Scholar Crossref - Ekman, Inger, et al. "Symptoms in patients with heart failure are prognostic predictors: insights from COMET." Journal of cardiac failure, Vol. 11, No. 4, 2005, pp. 288-92.
Google Scholar Crossref - Lechat, Philippe, et al. "Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial." Circulation, Vol. 103, No. 10, 2001, pp. 1428-33.
Google Scholar Crossref - Kitai, Takeshi, et al. "Insufficient reduction in heart rate during hospitalization despite beta‐blocker treatment in acute decompensated heart failure: insights from the ASCEND‐HF trial." European journal of heart failure, Vol. 19, No. 2, 2017, pp. 241-49.
Google Scholar Crossref - McAlister, Finlay A., et al. "Meta-analysis: β-blocker dose, heart rate reduction, and death in patients with heart failure." Annals of Internal Medicine, Vol. 150, No. 11, 2009, pp. 784-94.
Google Scholar Crossref - Cullington, Damien, et al. "Heart rate achieved or beta‐blocker dose in patients with chronic heart failure: which is the better target?." European journal of heart failure, Vol. 14, No. 7, 2012, pp. 737-47.
Google Scholar Crossref - Nikolaidis, Lazaros A., et al. "The effects of combined versus selective adrenergic blockade on left ventricular and systemic hemodynamics, myocardial substrate preference, and regional perfusion in conscious dogs with dilated cardiomyopathy." Journal of the American College of Cardiology, Vol. 47, No. 9, 2006, pp. 1871-81.
Google Scholar - DiBona, Gerald F. "Physiology in perspective: the wisdom of the body. Neural control of the kidney." American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, Vol. 289, No. 3, 2005, pp. R633-R41.
Google Scholar Crossref - Gardetto, Nancy J., and Karen C. Carroll. "Management strategies to meet the core heart failure measures for acute decompensated heart failure: a nursing perspective." Critical care nursing quarterly, Vol. 30, No. 4, 2007, pp. 307-20.
Google Scholar Crossref - Sliwa, Karen, et al. "Impact of initiating carvedilol before angiotensin-converting enzyme inhibitor therapy on cardiac function in newly diagnosed heart failure." Journal of the American College of Cardiology, Vol. 44, No. 9, 2004, pp. 1825-30.
Google Scholar Crossref - Kociol, Robb D., et al. "Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure." Circulation: Heart Failure, Vol. 6, No. 2, 2013, pp. 240-45.
Google Scholar Crossref - Lala, Anuradha, et al. "Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF)." Circulation: Heart Failure, Vol. 8, No. 4, 2015, pp. 741-48.
Google Scholar Crossref - Mehta, Rajendra H., et al. "Association of weight change with subsequent outcomes in patients hospitalized with acute decompensated heart failure." The American journal of cardiology, Vol. 103, No. 1, 2009, pp. 76-81.
Google Scholar Crossref - Nikolaidis, Lazaros A., et al. "The effects of combined versus selective adrenergic blockade on left ventricular and systemic hemodynamics, myocardial substrate preference, and regional perfusion in conscious dogs with dilated cardiomyopathy." Journal of the American College of Cardiology, Vol. 47, No. 9, 2006, pp. 1871-81.
Google Scholar Crossref - Tamaki, Yodo, et al. "Lower In‐Hospital Mortality With Beta‐Blocker Use at Admission in Patients With Acute Decompensated Heart Failure." Journal of the American Heart Association, Vol. 10, No. 13, 2021.
Google Scholar Crossref - Rivas, Francisco J., et al. "Carvedilol in Patients with Acutely Decompensated Systolic Heart Failure: Effects on Survival." American Journal of Internal Medicine, Vol. 9, No. 4, 2021. pp. 186-93.
Google Scholar