Research - International Journal of Medical Research & Health Sciences ( 2021) Volume 10, Issue 5
Evaluation of Efficacy of Locally Delivered 10% Azadirachta indica (Neem) Gel as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis Patients with Type II Diabetes Mellitus: Clinico-Microbiological Study
Shretika S. Pandya1, Anita M. Kulloli1, Sharath K. Shetty1, Santosh S. Martande1, Shantanu B. Pharande1, Swathi PV2* and Amol Deodhar12Department of Orthodontics and Dentofacial Orthopedics, Dr. D. Y. Patil Vidyapeeth, Pune, India
Swathi PV, Department of Orthodontics and Dentofacial Orthopedics, Dr. D. Y. Patil Vidyapeeth, Pune, India, Email: swathi.v@dpu.edu.in
Received: 21-Apr-2021 Accepted Date: May 18, 2021 ; Published: 25-May-2021
Abstract
Introduction: Increased prevalence and severity of periodontitis is seen in patients with Diabetes Mellitus (DM). This relationship is evident in patients with chronic periodontitis and poorly controlled diabetes. Lately, A. indica has been sh own to have anti-plaque activity. A. indica has shown remarkable improvements in the blood glucose levels signifying a possible hypoglycemic effect. The present study evaluated the clinical and microbiological effect of locally delivered 10% A. indica gel in the treatment of chronic periodontitis with Type II diabetes mellitus patients.
Materials and methods: A total of 15 chronic periodontitis with Type II Diabetes mellitus patients were included in the study. 30 sites from 15 patients were selected according to split-mouth design. Control group: (n=15) SRP alone. Test group: (n=15) SRP+A. indica gel. At the selected sites, clinical parameters were recorded at day 0, 6 weeks, and 3 months post-therapy. Plaque samples were collected at baseline and 3 months post-therapy for microbiological evaluation of Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Capnocytophaga, and Aggregatibacter actinomycemetemcomitans by multiplex Polymerase Chain Reaction (PCR). HbA1c levels were noted at baseline and 3 months.
Results: All clinical parameters showed a statistically significant difference in their baseline and 3 months (intragroup) comparison while in the comparison between the groups i.e. control and test group only PPD, RAL showed statistical significance, while PI, GI, RGML did not show a statistically significant difference. The HbA1 levels showed a statistically significant difference at 3 months. In the microbiological evaluation, all bacteria in both control and test groups at baseline and 3 months post-therapy showed statistical significance except Tannerella forsythia did not show a statistically significant difference. Intergroup comparison was statistically not significant.
Conclusion: The present study concluded that 10% A. indica gel can be effectively used as an adjunct to SRP in the treatment of chronic periodontitis with type II DM patients and for the reduction of microbial load in the subgingival environment.
Keywords
Chronic periodontitis, Local drug delivery, Neem, A. indica, Herbal agents
Introduction
Chronic Periodontitis (CP) is inflammations of periodontium induced by microbes, leading to gingival inflammation, periodontal tissue breakdown, and alveolar bone loss, and ultimately tooth loss [1]. Increased prevalence and severity of periodontitis are seen in patients with Diabetes Mellitus (DM). This relationship is evident in patients with chronic periodontitis and poorly controlled diabetes. CP has therefore been considered as the sixth complication of DM [2].
Reducing the microbial load is the ultimate goal of conventional periodontal therapy. However, it does not eliminate all the pathogens which reside deep into the connective tissue [3].
Antimicrobial agents are therefore used systemically or locally as an adjunct to periodontal therapy in the management of chronic periodontitis. These agents aim to reinforce mechanical periodontal treatment and to support the host defense system in overcoming the infection by killing subgingival pathogens that remain after conventional mechanical periodontal therapy [3].
The primary rationale for local drug delivery was to place the antimicrobial agent in direct contact with the root surface, so that the microorganisms inaccessible to mechanical debridement may be reduced or eliminated. They also establish a drug reservoir in the periodontal pocket that could maintain effective concentrations at the site of action for longer periods despite a loss from crevicular fluid clearance.
Various locally delivered agents that are successfully used include tetracycline, doxycycline, chlorhexidine, minocycline, azithromycin, etc. as an adjunct to scaling and root planing [4-8]. Of late, many natural products are used for the treatment of periodontitis due to the multitude of benefits they offer, their ease of application, rapid availability, and no/limited side effects.
Various locally delivered agents that are successfully used include tetracycline, doxycycline, chlorhexidine, minocycline, azithromycin, etc. as an adjunct to scaling and root planing [4-8]. Of late, many natural products are used for the treatment of periodontitis due to the multitude of benefits they offer, their ease of application, rapid availability, and no/limited side effects.
Among the various currently available natural or herbal agents, Azadirachta indica (A. indica) is considered to be a promising and high-ranking agent. Azadirachta indica (neem) has been used since ancient times for medicinal purposes. It has been proven to have antimicrobial, anti-inflammatory, antioxidant, antiseptic, and astringent properties [13].
A. indica has shown remarkable improvements in blood glucose levels signifying a possible hypoglycemic effect. The reduction in the fasting glucose and Glycosylated hemoglobin (HbA1c) levels post-neem use showed that it may safely improve glycemic control. Hence, treatment with A. indica (neem) may aid in the normalization of blood glucose and stimulate the body’s antioxidant defenses [14].
In dentistry, A. indica in various forms has been used since ancient times for oral diseases. Lately, A. indica has been shown to have anti-plaque activity [15]. It has also been shown to be effective for the treatment of chronic gingivitis [16].
Due to the above-known properties, A. indica has been used as a local drug delivery agent in Chronic Periodontitis and its gel form has shown improvement in Plaque Index (PI), Gingival Index (GI), and Probing Pocket Depth (PPD) [3].
Hence, the present study was designed to evaluate the clinical and microbiological effect of locally delivered A. indica gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis patients with Type II diabetes mellitus.
Material and Methods
The study population consisted of 30 sites from 15 chronic periodontitis patients with Type II Diabetes Mellitus, equal males, and females in the age group of 30-55 years were selected based on the following criteria. The study was approved by the Institution Ethics Committee. The procedure was explained and written informed consent was obtained from the participants before the study.
Inclusion Criteria
• The age group of 30 to 55 years from both genders (equal sex ratio)
• Patients should have at least 14 natural teeth in the oral cavity
• Patients with moderately controlled type-II diabetes mellitus and glycated hemoglobin A1c (HbA1c%) in the range of 7%-8%.
• Patients agreeing to sign the informed consent and willing to return for follow-up visits
• Chronic periodontitis patients should have Gingival index (GI) ≥ 1, Plaque index (PI) ≥ 1, Probing Pocket Depth (PPD) ≥ 5mm, Clinical Attachment Level (CAL) ≥ 3mm, Evidence of radiographic bone loss on OPG
Exclusion Criteria
• Patients with a known history of systemic diseases as hypertension, Human Immunodeficiency Virus (HIV), bone disorders, renal disorders, radiation therapy, cancer patients, infectious diseases, and any other systemic disease that can alter the course of periodontal disease
• History of prolonged use of steroids/immunosuppressive agents/anticoagulants
• Patients with antimicrobial therapy within the previous 3 months
• Patients with aggressive periodontitis
• Patients who have received periodontal therapy in the preceding 6 months
• Type-1 Diabetes Mellitus
• Pregnant/Lactating women
• History of tobacco in any form
A total of 30 sites from 15 chronic periodontitis patients with type II diabetes mellitus with at least one pair of deepest periodontal pockets in the contralateral quadrant were selected. The sample size was determined based on the power analysis at a confidence level of 95% (p?0.05). In each patient, one pair of periodontal pocket was randomly divided into 2 groups.
Control group: (n=15) Scaling and Root Planing (SRP) alone.
Test group: (n=15) Scaling and root planing followed by application of 10% A. indica gel (SRP+A. indica gel).
The following clinical parameters were recorded at baseline, 6 weeks, and 3 months post-therapy. Plaque Index (PI), Gingival Index (GI), Probing Pocket Depth (PPD), Relative Attachment Level (RAL), Relative Gingival Margin Level (RGML) [2].
All clinical parameters were recorded at baseline, 6 weeks, and 3 months post-therapy whereas HbA1c% levels were recorded at baseline and 3 months post-therapy.
Plaque samples were collected from the same site at baseline and 3 months post-therapy for microbial evaluations of Porphyromonas gingivalis, Trannerella forsythia, Treponema denticola, Aggregatibacter actinomycemetemcomitans, and Capnocytophaga by multiplex polymerase chain reaction.
Gel Constituents
• indica extract: 10% w/w-2 gm
• Carbopol 974: 1% w/w-0.2 gm
• Methyl parabaen: 0.02% w/w 0.036 gm
• Propyl paraben: 0.18% w/w-0.004 gm
• Water (distilled)-q.s
• Triethanolamine-for adjusting pH to 6.8
Clinical Procedure
The supragingival plaque was removed and contamination with saliva was avoided by isolation with cotton rolls and air-dried. Subgingival plaque samples were collected from the deepest periodontal pocket from the control and test sites for each patient using Sterile No. 40 paper points. One paper point was used for one site and was kept in situ for 30 seconds for saturation.
The samples were stored in Reduced Transport Fluid (RTF) medium and were sent for microbiological evaluation of Porphyromonas gingivalis, Trannerella forsythia, Treponema denticola, Aggregatibacter actinomycemetemcomitans, and Capnocytophaga using multiplex Polymerase Chain Reaction (PCR).
Control group: Full mouth supragingival and subgingival Scaling and Root Planing (SRP) was performed, with the help of hand-activated and power-driven instruments like curettes and ultrasonic scalers until the root surface appears to be clean and smooth. The patient was recalled after 4 weeks. If the pockets persisted the area was irrigated with normal saline and a periodontal dressing was placed which was removed after 7 days.
Test group: Full mouth supragingival and subgingival Scaling and Root Planing (SRP) was performed, with the help of hand-activated and power-driven instruments like curettes and ultrasonic scalers until the root surface appears to be clean and smooth. The patient was recalled after 4 weeks. If the pockets persisted, 10% A. indica gel was placed, using a syringe and a blunt needle, till the pocket is overfilled leaving a residue visible at the gingival margin of the test site. The periodontal dressing was placed to ensure that the gel remains in place. The dressing was removed after 7 days.
No medications (e.g. antibiotics, anti-inflammatory, or antiplaque agents) were prescribed after treatment.
Post Therapy Care and Follow Up
For the time the periodontal dressing was placed the patients were advised not to brush in the area. After which they were advised to use a soft-bristled toothbrush with a fluoridated toothpaste and brush twice daily employing the modified Bass technique.The PI, GI, PPD, RAL, and RGML were repeated at 6 weeks and 3 months post-therapy in both groups, while HbA1c% was done at baseline and 3 months.
Microbial Evaluation
The plaque samples were collected at baseline and 3 months post-therapy from the deepest periodontal pockets from control and test groups. They were sent for microbiological evaluation of Porphyromonas gingivalis, Trannerella forsythia, Treponema denticola, Aggregatibacter actinomycemetemcomitans, and Capnocytophaga using multiplex PCR (semi-quantitative analysis)
Statistical Evaluation
Power calculations were performed before the study; power calculations were initiated to achieve 95% power. The mean intergroup and intragroup comparisons for all clinical parameters i.e. PI, GI, PPD, RAL, RGML were performed using the Unpaired ‘t’ test and repeated measures Analysis of Variance (ANOVA), respectively. The difference between each pair of measurements was calculated (baseline, 6 weeks, and 3 months). Unpaired ‘t’ test and Paired ‘t’ test was applied to assess the statistical significance of microbiological evaluation for intragroup and intergroup comparison. Statistical significance was considered significant for a p-value of less than 0.05 (p ≤ 0.05).
Results
Plaque Index (PI): The mean plaque index score at baseline for the control group and test group was 2.013 ± 0.15 and 2.087 ± 0.30 respectively. At 6 weeks it reduced to 1.100 ± 0.25, showing a reduction of 0.913 ± 0.06 for the control group and 1.107 ± 0.33 showing a reduction of 0.980 ± 0.026 for the test group. At 3 months it reduced to 0.607 ± 0.13 showing a reduction of 1.407 ± 0.039 for the control group and 0.600 ± 0.19 with a reduction of 1.48 ± 0.018, showed statistically significant difference (p<0.001). Comparison between PI scores of control and test group at baseline, 6 weeks, and 3 months showed no statistically significant difference p?0.41 and p?0.95 and p?0.91 respectively (Table 1).
| Control | Test | p-value | ||
|---|---|---|---|---|
| Plaque Index | Baseline | 2.013 ± 0.15 | 2.087 ± 0.30 | 0.41 |
| 6 weeks | 1.100 ± 0.25 | 1.107 ± 0.33 | 0.95 | |
| 3 months | 0.607 ± 0.13 | 0.600 ± 0.19 | 0.91 | |
| 0.001* | 0.001* | |||
| Gingival Index | Baseline | 2.127 ± 0.28 | 2.153 ± 0.28 | 0.79 |
| 6 weeks | 1.193 ± 0.32 | 1.200 ± 0.33 | 0.95 | |
| 3 months | 0.600 ± 0.14 | 0.627 ± 0.23 | 0.71 | |
| 0.001* | 0.001* | |||
| Probing Pocket Depth | Baseline | 9.88 ± 1.64 | 10 ± 1.4 | 0.81 |
| 6 weeks | 8.2 ± 1.37 | 6.6 ± 1.4 | 0.006* | |
| 3 months | 7.0 ± 1.25 | 4.73 ± 1.09 | 0.00001* | |
| 0.001* | 0.001* | |||
| Clinical Attachment Levels | Baseline | 12.5 ± 1.8 | 13.4 ± 1.8 | 0.204 |
| 6 weeks | 11.3 ± 1.67 | 9.8 ± 1.6 | 0.01* | |
| 3 months | 10.1 ± 1.5 | 7.8 ± 1.5 | 0.0004* | |
| 0.001* | 0.001* | |||
| Relative Gingival Marginal levels | Baseline | 2.6 ± 0.61 | 3.0 ± 1.05 | 0.2 |
| 6 weeks | 3.13 ± 0.74 | 3.13 ± 0.6 | 1 | |
| 3 months | 3.13 ± 0.95 | 3.13 ± 0.95 | 1 | |
| 0.007* | 0.05* | |||
*p ≤ 0.05 is considered to be statistically significant
Table 1: Comparison of plaque index, gingival index, probing pocket depth, relative attachment levels, and relative gingival marginal levels of control and test group at baseline, 6 weeks and 3 months
Gingival Index (GI): The mean gingival index score for the control group at baseline was 2.127 ± 0.28. At 6 weeks was 1.193 ± 0.32 showing a reduction of 0.93 ± 0.026 and at 3 months 0.600 ± 0.14 which showed a reduction of 1.5 ± 0.018. The gingival index scores showed a statistically significant difference (p<0.001). The mean gingival index score at baseline for the test group was 2.153 ± 0.28 at 6 weeks was 1.200 ± 0.33 showing a reduction of 0.95 ± 0.06 and at 3 months 0.627 ± 0.23 which showed a reduction of 1.5 ± 0.018. The gingival index scores showed a statistically significant difference (p<0.001). Comparison between GI scores of control and test group at baseline, 6 weeks, and 3 months showed no statistically significant difference p?0.79 and p?0.95 and p?0.71 respectively (Table 1).
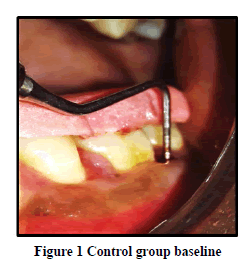
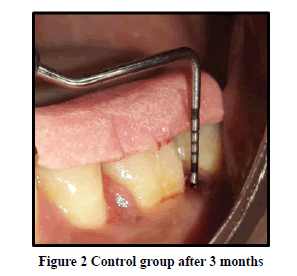
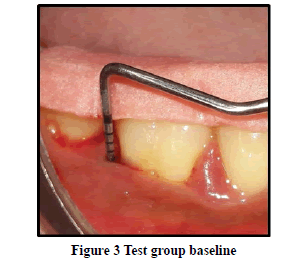
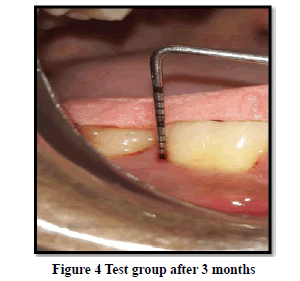
Probing Pocket Depth (PPD): The mean probing pocket depth at baseline for the control group was 9.88 ± 1.64 mm (Figure 1). At 6 weeks PD reduced to 8.2 ± 1.37 mm showing a reduction of 1.68 ± 0.07 mm and at 3 months PD reduced to 7.0 ± 1.25 mm showing a reduction of 2.88 ± 0.05 mm showed statistically significant difference (p<0.001) (Figure 2). The mean probing pocket depth at baseline for the test group was 10 ± 1.46 mm (Figure 3). At 6 weeks PD reduced to 6.6 ± 1.49 mm showing a reduction of 3.4 ± 0.25 mm and at 3 months PD reduced to 4.73 ± 1.09 mm showing a reduction of 5.3 ± 0.38 mm showed statistically significant difference (p<0.001) (Figure 4). Comparison between PD of control and test group at baseline was not statistically significant p>0.81 and 6 weeks and 3 months it was statistically significant with p-value p<0.006 and p<0.00001 respectively (Table 1).
Relative Attachment Level (RAL): The mean relative attachment level at baseline for the control group was 12.5 ± 1.8 mm (Figure 1). At 6 weeks RAL reduced to 11.3 ± 1.67 mm showing a gain of 1.2 ± 0.02 mm and at 3 months RAL reduced to 10.1 ± 1.5 mm showing a gain of 2.4 ± 0.07 mm showed statistically significant difference (p<0.001) (Figure 2). The mean relative attachment level at baseline for the test group was 13.4 ± 1.8 mm (Figure 3). At 6 weeks RAL reduced to 9.8 ± 1.6 mm showing a gain of 3.6 ± 0.09 mm and at 3 months RAL reduced to 7.8 ± 1.5 mm showing a gain of 5.6 ± 0.26 mm showed statistically significant difference (p<0.001) (Figure 4). Comparison between RAL of control and test group at baseline was not statistically significant p>0.204 and 6 weeks and 3 months it was statistically significant p<0.01 respectively p<0.0004 (Table 1).
Relative Gingival Margin Level (RGML): The mean Relative Gingival Margin level at baseline for the control group was 2.6 ± 0.61 mm (Figure 1). At 6 weeks, this distance increased to 3.13 ± 0.74 mm, showing a gingival recession of 0.53 ± 0.06 mm. At 3 months RGML was remained the same at 3.13 ± 0.95 mm, showing no further recession which was a statistically significant difference (p<0.007) (Figure 2). The mean Relative Gingival Margin level at baseline for the test group was 3.0 ± 1.05 mm (Figure 3). At 6 weeks, this distance increased to 3.13 ± 0.6 mm, showing a gingival recession of 0.13 ± 0.06 mm. At 3 months RGML was remained the same at 3.13 ± 0.95 mm, showing no further recession which was a statistically significant difference (p<0.05) (Figure 4). Comparison between RGML of control and test group there was no statistically significant difference at baseline p>0.2, 6 weeks p>1 and 3 months p>1 respectively (Table 1).
Glycated Hemoglobin (HbA1c%) Levels: The mean HbA1c% levels at baseline was 7.51 ± 0.344 which reduced to 7.39 ± 0.375 at 3 months with a mean difference of 0.12 ± 0.03 which showed statistically significant difference with p<0.048 (Table 2).
| Baseline | 3 months | p-value | |
|---|---|---|---|
| HbA1c | 7.51 ± 0.344 | 7.39 ± 0.375 | 0.048* |
*p ≤ 0.05 is considered to be statistically significant
Table 2: Comparison of hba1c at baseline and 3 months
Microbiological Evaluation
Porphyromonas gingivalis: The mean DNA copies/ml of Porphyromonas gingivalis at baseline for the control group was 19.3 ×109± 25.3 × 109 and reduced to 15.6 × 102 ± 24.5 × 102 at 3 months post-therapy showing a statistically significant difference (p<0.03) (Figure 5, and Figure 6). The mean DNA copies/ml of Porphyromonas gingivalis at baseline for the test group was 12.4 × 109 ± 22.9 × 109 and reduced to 10.1 × 103 ± 25.7 × 103 at 3 months post-therapy showing a statistically significant difference (p<0.03) (Figure 5, and Figure 6). Comparison between DNA copies/ml of the control group and test group at baseline and 3 months post-therapy showed no statistical significance difference for Porphyromonas gingivalis p>0.4 and p>0.2 respectively (Table 3).
| Control | Test | p-value | ||
|---|---|---|---|---|
| Porphyromonas gingivalis | Baseline | 19.3 × 109 ± 25.3 × 109 | 12.4 × 109 ± 22.9 × 109 | 0.4 |
| 3 months | 15.6 × 102 ± 24.5 × 102 | 10.1 × 103 ± 25.7 × 103 | 0.2 | |
| 0.03* | 0.03* | |||
| Treponema denticola | Baseline | 12.6 × 109 ± 21.3 × 109 | 15.6 × 109 ± 21.2 × 109 | 0.7 |
| 3 months | 17.4 × 102 ± 20.7 × 102 | 22.0 × 102 ± 27.8 × 102 | 0.6 | |
| 0.03* | 0.01* | |||
| Tannerella forsythia | Baseline | 59.3 × 107 ± 17.0 × 108 | 25.6 × 107 ± 84.2 × 108 | 0.2 |
| 3 months | 46.2 × 101 ± 14.8 × 102 | 43.5 × 101 ± 13.5 × 102 | 0.3 | |
| 0.25 | 0.12 | |||
| Capnocytophaga | Baseline | 16.3 × 108 ± 20.8 × 109 | 10.4 × 109 ± 18.2 × 109 | 0.4 |
| 3 months | 34.1 × 102 ± 16.8 × 102 | 30.5 × 102 ± 20.1 × 102 | 0.5 | |
| 0.043* | 0.009* | |||
| Aggregatibacter actinomycemetemcomitans | Baseline | 12.3 × 109 ± 20.4 × 109 | 12.4 × 109 ± 19.4 × 109 | 0.9 |
| 3 months | 16.2 × 102 ± 33.1 × 102 | 19.52 × 102 ± 30.1 × 102 | 0.7 | |
| 0.01* | 0.04* | |||
*p ≤ 0.05 is considered to be statistically significant
Table 3: Comparison of micro-organisms of control and test groups at baseline and 3 months
Treponema denticola: The mean DNA copies/ml of Treponema denticola at baseline for the control group was 12.6 ×109± 21.3 × 109 and reduced to 17.4 ×102± 20.7 × 102 at 3 months post-therapy showing a statistically significant difference (p<0.03) (Figure 5, and Figure 6). The mean DNA copies/ml of Treponema denticola at baseline for the test group was 15.6 ×109± 21.2 × 109 and reduced to 22.0 ×102± 27.8 × 102 at 3 months post-therapy showing a statistically significant difference (p<0.01) (Figure 5, and Figure 6). Comparison between DNA copies/ml of the control group and test group at baseline and 3 months post-therapy showed no statistical significance difference for Tannerella forsythia p>0.2 and p>0.3 respectively (Table 3).
Capnocytophaga: The mean DNA copies/ml of Capnocytophaga at baseline for the control group was 16.3 × 108± 20.8 × 109 and reduced to 34.1 × 102 ± 16.8 × 102 at 3 months post-therapy showing a statistically significant difference (p<0.043). The mean DNA copies/ml of Capnocytophaga at baseline for the test group was 10.4 ×109± 18.2 × 109 and reduced to 30.5 ×102± 20.1 × 102 at 3 months post-therapy showing a statistically significant difference (p<0.009) (Table 3).
Comparison between DNA copies/ml of the control group and test group at baseline and 3 months post-therapy showed no statistical significance difference for Capnocytophaga p>0.4 and p>0.5 respectively.
Aggregatibacter actinomycemetemcomitans: The mean DNAcopies/ml of Aggregatibacter actinomycemetemcomitans at baseline for the control group was 12.3 ×109± 20.4 × 109 and reduced to 16.2 ×102± 33.1 × 102 at 3 months post- therapy showing a statistically significant difference (p<0.01) (Figure 5, and Figure 6). The mean DNA copies/ml of Aggregatibacter actinomycemetemcomitans at baseline for the test group was 12.4 ×109± 19.4 × 109 and reduced to 19.52 ×102± 30.1 × 102 at 3 months post-therapy showing a statistically significant difference (p<0.04) (Figure 5, and Figure 6). Comparison between DNA copies/ml of the control group and test group at baseline and 3 months post-therapy showed no statistical significance difference for Aggregatibacter actinomycemetemcomitans p>0.9 and p>0.7 respectively (Table 3).
Discussion
Several studies have evaluated the effect of periodontal mechanical therapy and glycemic control in diabetes mellitus patients have noted a 17.1% improvement in the glycemic control of CP patients who received conventional periodontal therapy as compared to the group of individuals who did not receive periodontal therapy [17-19].
It was suggested by Goodson, et al. that as the pocket depth progressively increases, scaling and root planing become less effective, and therefore the use of adjunctive treatments along with mechanical therapy was advocated and hence, in patients with systemic disease, adjunctive treatment of antimicrobials is indicated [20-22].
Amongst the herbal drugs, A. indica (neem) is considered to be the most promising and high-ranking agent. A. indica is known to have various therapeutic properties i.e. anti-inflammatory, antibacterial, anti-hyperglycemic, etc. A. indica when administered systemically has been shown to reduce HbA1c levels in type II diabetes mellitus patients [14,23,24].
A. indica is known to have a microbial growth/potentiality of cell wall breakdown. It is also known to have a significant stimulatory effect on insulin secretion [25].
Studies by Wolinsky, Pai, Chaterjee have shown that A. indica is effective clinically and microbiologically in the management of CP [15,26,27]. A. indica has shown superior anti-hyperglycemic activity in animals, in vitro, and in vivo. It reduces pancreatic and intestinal glucosidase. Digestion of dietary carbohydrates is brought about by glucosidase that is present in the pancreas and gut of the small intestine. The extracts retarded the glucosidase activity which helps in controlling blood glucose levels. It can also reactivate the glycogen synthase systems.
CP patients with moderately controlled type II DM with HbA1c 7 to 8 were selected for the study because in most cases, well-controlled DM patients can be managed similar to that in healthy non-diabetic patients, whereas uncontrolled diabetics are more susceptible to infections and other health complications which would require alterations in medications or lifestyle [28]. Such patients also demonstrate a quick decrease in HbA1c level after non-surgical periodontal therapy compared to well-controlled diabetic patients with a “rebound effect” of the HbA1c back to baseline level [17].
In our study, 10% A. indica in the gel form was used as it contains carbopol. Carbopol is a gelling agent and has mucoadhesive properties which help the drug to adhere to the mucosa for a longer duration and thus prevent its ‘washing off’ due to the action of saliva and Gingival Crevicular Fluid (GCF).
In the present study, there was a significant reduction in PI and GI scores in both control and test groups at 6 weeks as well as at 3 months compared to baseline. The comparison of mean PI and GI scores between the two groups was statistically not significant. Our results are following the studies by Venilla, et al. and Antony VV, et al. [3,9]. These findings may be attributed to thorough mechanical periodontal therapy along with patient motivation and oral hygiene maintenance with periodic recall visits.
In the intragroup analysis, the present study showed that the mean probing pocket depth at baseline in the control group was 9.88 ± 1.64 mm and at 3 months PPD reduced to 7.0 ± 1.25 mm showing a reduction of 2.88 ± 0.05 mm (p<0.001). The mean probing pocket depth in the test group at baseline was 10 ± 1.46 mm and at 3 months PPD reduced to 4.73 ± 1.09 mm showing a reduction of 5.3 ± 0.38 mm (p<0.001).
In our study, the intragroup analysis of the mean relative attachment level in the control group at baseline was 12.5 ± 1.8 mm and at 3 months RAL was reduced to 10.1 ± 1.5 mm showing a gain of 2.4 ± 0.07 mm showed statistically significant difference (p<0.001). The mean relative attachment level for the test group at baseline was 13.4 ± 1.8 mm and at 3 months RAL reduced to 7.8 ± 1.5 mm showing a gain of 5.6 ± 0.26 mm showed statistical significance (p<0.001).
Intergroup comparison between PPD of control and test group at baseline was statistically not significant (p>0.81) and 6 weeks and 3 months it was statistically significant with p<0.006 respectively p<0.00001 respectively. Intergroup comparison between RAL of control and test group at baseline was statistically not significant with p>0.204 and 6 weeks and 3 months it was statistically significant with p<0.01 and p<0.0004 respectively.
Our study is following the study by Jain, et al. also showed a statistically significant reduction in PD of 2.40 ± 0.14 mm and a gain in CAL of 1.7 ± 0.9 mm when compared to scaling and root planing alone and scaling and root planing with A. indica as an adjunct [29]. These findings are also per a study done by Pradeep AR who compared Aloe vera gel as an adjunct to scaling and root planing versus scaling and root planing alone in type II DM patients and found a reduction in PPD of 1.83 ± 0.37 mm and a CAL gain of 1.07 ± 0.25 mm [1].
SRP leads to reduction of periodontal pathogens, resolution of inflammation, tissue shrinkage and also provides a smooth surface of the root surface which aids in the attachment of gingival connective tissue and epithelium [30,31].
The addition of 10% A. indica gel which is known to have superior antibacterial and anti-inflammatory properties against periodontal pathogens and also accelerates wound healing as it contains an excellent amount of amino acid, vitamin, and mineral that are important in wound healing processes in the proliferation phase [15]. Hence, the test group showed a statistical difference when compared to the control group.
The mean RGML at baseline for the control group was 2.6 ± 0.61 mm. At 6 weeks, this distance increased to 3.13 ± 0.74 mm, showing a gingival recession of 0.53 ± 0.06 mm. At 3 months RGML was remained the same at 3.13 ± 0.95 mm, showing no further recession which was statistical significance (p<0.007). The mean RGML at baseline for the test group was 3.0 ± 1.05 mm. At 6 weeks, this distance increased to 3.13 ± 0.6 mm, showing a gingival recession of 0.13 ± 0.06 mm. At 3 months RGML was remained the same at 3.13 ± 0.95 mm, showing no further recession which was statistically significant (p<0.05). Intragroup comparison at baseline, 6 weeks, and 3 months was not statistically significant with p?0.2, p?1, and p?1 respectively. The change could have been contributed to a reduction in PPD.
In our study gingival recession is seen in both control and test groups because scaling and root planing lead to resolution of the gingival inflammation, which causes tissue shrinkage and recession of the gingival margin. The more the severity of the inflammation, there is greater the tendency for gingival recession [32].
Furthermore, HbA1c levels were recorded in all patients at baseline and 3 months. HbA1c is amongst the most important and reliable tests for DM patients which are used to evaluate the severity of the condition [17].
The binding of hemoglobin from the erythrocytes to glucose is indicated as HbA1c levels and hence alterations in HbA1c levels are measured during half of the life span of erythrocyte cells (30-90 days). Therefore these levels were re-evaluated, 3 months after periodontal therapy.
The mean HbA1c% levels at baseline were 7.51 ± 0.344 which reduced to 7.39 ± 0.375 at 3 months with a mean difference of 0.1200 ± 0.03 which showed statistical significance (p<0.048). The change seen may be due to the reduction of micro-organisms and inflammation after the therapy. The decrease in local periodontal inflammation reduces the levels of pro-inflammatory cytokines which are implicated to impair insulin signaling and resistance [17]. This was further maintained by oral hypoglycemic medications taken by the patients. These findings are under the meta-analysis systematic review by Sanz, et al. who stated that there is a consistent and statistically significant reduction in the HbA1c levels after non-surgical periodontal therapy in patients with type II DM [17].
A. indica has anti-diabetic effects and is known to reduce blood glucose levels. Hence A. indica could have played an adjunctive role in the reduction of HbA1c levels. However, since this is a split-mouth study, the reduction of HbA1c levels was because the combined treatment or scaling and root planing alone cannot be evaluated.
A. indica shows its antimicrobial properties by inhibiting microbial growth and it also knows to break down the cell wall this acting as a bactericidal and a bacteriostatic agent. It is also known to be a potent antioxidant by scavenging free radicals. The anti-inflammatory activity is brought about by regulating the pro-inflammatory enzymes like the Cyclooxygenase (COX), and Lipoxygenase (LOX) enzyme. Nimbidin, an active constituent in A. indica suppresses the function of macrophages and neutrophils involved in inflammation. The anti-hyperglycemic actions of A. indica are due to decreased activities of Superoxide Dismutase (SOD) and Catalase (CAT) brought about by A. indica and thus increase in Lipid Peroxidation (LPO) observed in the erythrocytes thus reducing oxidative stress. It also acts via stimulating the effect of insulin [33-35].
In chronic periodontitis patients, Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, and Aggregatibacter actinomycemetemcomitans are detected in high frequency. However, diabetes causes a rise in the concentration of glucose in the saliva and gingival crevicular fluid and contributes to the alteration in the microbial profile in the periodontal pocket. Clinical investigations have demonstrated that this modified environment is more favorable for the growth of Gram-negative anaerobic bacteria including organisms like Capnocytophaga, black- pigmented bacteria, etc. [36]. Hence, in our study, Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Capnocytophaga, and Aggregatibacter actinomycemetemcomitans, were evaluated at baseline and 3 months post-therapy using multiplex polymerase chain reaction.
Our baseline microbial analysis of the micro-organisms is in congruence with studies carried out by Silva-Boghossian, et al. Mandell, et al. and Cruz, et al. who have all detected a high frequency of Porphyromonas gingivalis, Treponema denticola, Capnocytophaga, Aggregatibacter actinomycemetemcomitans but low frequency of Tanerella forsythia in their studies [37-39].
The present study showed a statistically significant decrease in the intragroup comparison in both the control and test groups, in Porphyromonas gingivalis, Treponema denticola, Capnocytophaga, Aggregatibacter actinomycemetemcomitans from baseline to 3 months, but not in Tanerella forsythia as they were detected in low frequency. While the intergroup comparison showed a reduction in the bacterial load but was not statistically significant.
Scaling and root planing alone may not be capable of eliminating micro-organisms in patients with CP with type II DM [37]. Studies have reported a reduction in the prevalence of these pathogens when SRP was used in combination with antimicrobials, however, they were not eliminated but had significantly reduced levels, which enabled a good clinical response to be obtained, at least at 3 months after treatment in both groups. Our study is by studies done by Silva-Boghossian, et al. and da Cruz, et al. [37,39].
Patients were asked about any side effects like discomfort, allergies, or inflammation and clinical assessment of the same was done at every visit. No side effects were noted post-therapy and at all visits.
Hence, with the concluding results of the present study, the clinical and microbiological impression is that 10%
A. indica significantly improves the periodontal health in moderately controlled diabetic patients with statistically significant improvement in the PI score, GI score, PPD, RAL, and HbA1c values compared to SRP alone.
Conclusion
In conclusion, within the limits of the present study, 10% A. indica gel seemed to be effective in the treatment of chronic periodontitis with type II diabetes mellitus and could be used as an adjunct to scaling and root planing for the clinical and microbial status. Also, A. indica seems to be cost-effective with no side effects and with several qualities like anti-inflammatory, anti-bacterial, anti-hyperglycemic, and wound healing properties which explain the rapid improvement seen in few months. Hence, many researchers are becoming interested in using A. indica as an adjunct to scaling and root planing. Multicenter, long-term evaluation is needed to confirm the findings in this study. Also, the use of pressure-sensitive probes would eliminate human error.
Declarations
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
References
- Pradeep, A. R., et al. "Adjunctive local delivery of Aloe vera gel in patients with type 2 diabetes and chronic periodontitis: A randomized, controlled clinical trial."Journal of Periodontology,Vol. 87, No. 3, 2016, pp. 268-74.
- Löe, Harald. "Periodontal disease: The sixth complication of diabetes mellitus."Diabetes Care,Vol. 16, No. 1, 1993, pp. 329-34.
- Vennila, K., S. Elanchezhiyan, and Sugumari Ilavarasu. "Efficacy of 10% whole Azadirachta indica (neem) chip as an adjunct to scaling and root planing in chronic periodontitis: A clinical and microbiological study."Indian Journal of Dental Research,Vol. 27, No. 1, 2016, pp. 15-21.
- Panwar, M., and S. H. Gupta. "Local drug delivery with tetracycline fiber: An alternative to surgical periodontal therapy."Medical Journal Armed Forces India,Vol. 65, No. 3, 2009, pp. 244-46.
- Ioannou, Ioannis, et al. "The effect of locally delivered doxycycline in the treatment of chronic periodontitis. A clinical and microbiological cohort study."Journal of Oral and Maxillofacial Research,Vol. 1, No. 4, 2010, p. e1.
- Soskolne, W. A., et al. "Sustained local delivery of chlorhexidine in the treatment of periodontitis: A multi-center study."Journal of Periodontology,Vol. 68, No. 1, 1997, pp. 32-38.
- Jain, Ritu, Faizuddin Mohamed, and M. Hemalatha. "Minocycline containing local drug delivery system in the management of chronic periodontitis: A randomized controlled trial."Journal of Indian Society of Periodontology,Vol. 16, No. 2, 2012, pp. 179-83.
- Pradeep, A. R., et al. "Local drug delivery of 0.5% azithromycin in the treatment of chronic periodontitis among smokers."Australian Dental Journal,Vol. 58, No. 1, 2013, pp. 34-40.
- Antony, Verdine Virginia."Evaluation of the efficacy of Azadirachta indica (neem) extract gel as a local drug delivery in the treatment of patients with chronic periodontitis-A double blind randomised clinical study." IOSR Journal of Pharmacy, Vol. 3, No. 4, 2013, pp.15-21.
- Behal, Roobal, et al. "Evaluation of local drug-delivery system containing 2% whole turmeric gel used as an adjunct to scaling and root planing in chronic periodontitis: A clinical and microbiological study."Journal of Indian Society of Periodontology,Vol. 15, No. 1, 2011, pp. 35-38.
- Nagata, Hideki, et al. "Effect of eucalyptus extract chewing gum on periodontal health: A double-masked, randomized trial."Journal of Periodontology,Vol. 79, No. 8, 2008, pp. 1378-85.
- Saito, M. V., et al. "Antibacterial activity of extracts from eucalyptus leaves on periodontopathic bacteria."Journal of Dental Health-Tokyo,Vol. 53, No. 5, 2003, pp. 585-91.
- Kumar, Rakesh, Simpi Mehta, and Seema R. Pathak. "Bioactive constituents of neem."Synthesis of Medicinal Agents from Plants, 2018, pp. 75-103.
- Kochhar, Anita, Neha Sharma, and Rajbir Sachdeva. "Effect of supplementation of Tulsi (Ocimum sanctum) and Neem (Azadirachta indica) leaf powder on diabetic symptoms, anthropometric parameters and blood pressure of non insulin dependent male diabetics."Studies on Ethno-Medicine,Vol. 3, No. 1, 2009, pp. 5-9.
- Chatterjee, Anirban, et al. "To evaluate the antigingivitis and antipalque effect of an Azadirachta indica (neem) mouthrinse on plaque induced gingivitis: A double-blind, randomized, controlled trial."Journal of Indian Society of Periodontology,Vol. 15, No. 4, 2011, pp. 398-401.
- Botelho, Marco Antonio, et al. "Efficacy of a mouthrinse based on leaves of the neem tree (Azadirachta indica) in the treatment of patients with chronic gingivitis: A double-blind, randomized, controlled trial."Journal of Medicinal Plants Research,Vol. 2, No. 11, 2008, pp. 341-46.
- Sanz, Mariano, et al. "Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology."Diabetes Research and Clinical Practice,Vol. 137, 2018, pp. 231-41.
- Polak, David, and Lior Shapira. "An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes."Journal of Clinical Periodontology,Vol. 45, No. 2, 2018, pp. 150-66.
- Iwamoto, Yoshihiro, et al. "The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes."Journal of Periodontology,Vol. 72, No. 6, 2001, pp. 774-78.
- Goodson, J. M., A. Haffajee, and S. S. Socransky. "Periodontal therapy by local delivery of tetracycline."Journal of Clinical Periodontology,Vol. 6, No. 2, 1979, pp. 83-92.
- Grossi, Sara G., and Robert J. Genco. "Periodontal disease and diabetes mellitus: A two-way relationship."Annals of Periodontology,Vol. 3, No. 1, 1998, pp. 51-61.
- Goodson, J. Max. "Antimicrobial strategies for treatment of periodontal diseases."Periodontology 2000,Vol. 5, No. 1, 1994, pp. 142-68.
- Elder, Charles, et al. "Randomized trial of a whole-system ayurvedic protocol for type 2 diabetes."Alternative Therapies in Health and Medicine,Vol. 12, No. 5, 2006, pp. 24-30.
- Kumari, D. Jalaja. "Hypoglycaemic effect of Moringa oleifera and Azadirachta indica in type 2 diabetes mellitus."Bioscan,Vol. 5, No. 20, 2010, pp. 211-14.
- Kaur, Lovedeep, et al. "Indian culinary plants enhance glucose-induced insulin secretion and glucose consumption in INS-1 β-cells and 3T3-L1 adipocytes."Food Chemistry,Vol. 129, No. 3, 2011, pp. 1120-25.
- Wolinsky, L. E., et al. "The inhibiting effect of aqueous Azadirachta indica (Neem) extract upon bacterial properties influencing in vitro plaque formation."Journal of Dental Research,Vol. 75, No. 2, 1996, pp. 816-22.
- Pai, M. Raveendra, Leelavathi D. Acharya, and N. Udupa. "Evaluation of antiplaque activity of Azadirachta indica leaf extract gel-A 6-week clinical study."Journal of Ethnopharmacology,Vol. 90, No. 1, 2004, pp. 99-103.
- Rees, Terry D. "Periodontal management of the patient with diabetes mellitus."Periodontology 2000,Vol. 23, 2000, pp. 63-72.
- Jain, Sanjeev, Harjit Kaur, and Sumeet Brar. "To evaluate the efficacy of neem chip as an adjunct to Scaling and Root Planing (SRP) in patients with periodontitis."Indian Journal of Dental Sciences,Vol. 4, No. 1, 2012, pp. 42-45.
- Haffajee, A. D., et al. "The effect of SRP on the clinical and microbiological parameters of periodontal diseases."Journal of Clinical Periodontology,Vol. 24, No. 5, 1997, pp. 324-34.
- Lavanchy, Daniel Lionel, M. Bickel, and P. C. Baehni. "The effect of plaque control after scaling and root planing on the subgingival microflora in human periodontitis."Journal of Clinical Periodontology,Vol. 14, No. 5, 1987, pp. 295-99.
- Gupta, Ira, and K. L. Vandana. "Alterations of the marginal soft tissue (gingival margin) following periodontal therapy: A clinical study."Journal of Indian Society of Periodontology,Vol. 13, No. 2, 2009, pp. 85-89.
- McCalla, G., et al. "Beta cell regenerating potential of Azadirachta indica (Neem) extract in diabetic rats."West Indian Medical Journal,Vol. 65, No. 1, 2016, pp. 13-17.
- Satyanarayana, K., et al. "Molecular approach to identify antidiabetic potential of Azadirachta indica."Journal of Ayurveda and Integrative Medicine,Vol. 6, No. 3, 2015, pp. 165-74.
- Ponnusamy, Sudha, et al. "Gedunin and azadiradione: Human pancreatic alpha-amylase inhibiting limonoids from neem (Azadirachta indica) as anti-diabetic agents."PloS One,Vol. 10, No. 10, 2015, p. e0140113.
- Ciantar, Marilou, et al. "Capnocytophaga spp. in periodontitis patients manifesting diabetes mellitus."Journal of Periodontology,Vol. 76, No. 2, 2005, pp. 194-203.
- Silva-Boghossian, Carina Maciel, et al. "Microbiological changes after periodontal therapy in diabetic patients with inadequate metabolic control."Brazilian Oral Research,Vol. 28, No. 1, 2014, pp. 1-9.
- Mandell, Robert L., et al. "Microbiology of healthy and diseased periodontal sites in poorly controlled insulin dependent diabetics."Journal of Periodontology,Vol. 63, No. 4, 1992, pp. 274-79.
- Cruz, Gabriela Alessandra da, et al. "Clinical and laboratory evaluations of non-surgical periodontal treatment in subjects with diabetes mellitus."Journal of Periodontology,Vol. 79, No. 7, 2008, pp. 1150-57.