Research - International Journal of Medical Research & Health Sciences ( 2021) Volume 10, Issue 3
Evaluation of Semi-Elemental Formulae vs. Polymeric Formulae as Exclusive Enteral Nutrition in Active Pediatric Crohn’s Disease in India
Lekha Sreedharan1*, Dhanasekhar Kesavelu2 and Anusuya Devi K32Gastroenterology, Apollo Children’s Hospitals, Chennai, India
3Nutrition and Dietetics, PSG College of Arts and Science, Coimbatore, India
Lekha Sreedharan, Clinical Dietetics, Apollo Children’s Hospitals, Chennai, India, Email: Lekha_vs@yahoo.com
Received: 23-Feb-2021 Accepted Date: Mar 23, 2021 ; Published: 30-Mar-2021
Abstract
Background and aim: Exclusive Enteral Nutrition (EEN) is an evidence-based therapy for pediatric Crohn’s Disease (CD). Various clinical studies are conducted to compare the efficacy of polymeric versus elemental formulae in CD. This is the first Indian prospective observational study comparing the therapeutic efficacy of Polymeric Formulae (PF) vs. Semi-Elemental Formulae (SEF) to maintain clinical remission in Pediatric CD. Method: Children with active CD, who commenced on EEN were enrolled. The cohorts were assessed nutritionally and their disease activity was measured using Pediatric Crohn’s disease activity Index. Data were recorded at baseline and after 8 weeks of post EEN. Result: Out of the 38 children recruited, 20 children were treated with SEF and 18 children with PF. 45% children and 50% children were well-nourished in the SEF and PF group respectively. One child relapsed from each of the cohorts within a year. Post therapy, 85% were well-nourished in the SEF group and 77.7% of children were wellnourished in the PF group. In both groups, significant weight gain (p<0.000) was noted at the end of EEN therapy. Conclusion: Polymeric formulae and Semi elemental formulae are effective in inducing clinical remission in active CD with no systemic side effects.
Keywords
Exclusive Enteral Nutrition (EEN), Pediatric Crohn’s disease, Polymeric Formulae (PF), Semi-Elemental Formulae (SEF)
Introduction
Crohn’s Disease (CD) is a chronic disease characterized by recurrent exacerbations and remissions. Nutritional therapy for CD has been effectively used since the condition was initial represented in 1932 [1]. The earliest reports of the use of nutritional therapy indicate that it had been used primarily as a method to boost nutrition in CD patients who were unfit for surgery. A high carbohydrate, high protein, low fiber diet with additional supplements for specific nutritional deficiencies was used in this therapy [2,3]. The potential effectiveness of a nutritional-based therapy for direct treatment of CD was first reported by surgeons in the 1970s [4]. Exclusive Enteral Nutrition (EEN) was found to be effective in inducing clinical remission in active pediatric CD with no systemic side effects in the first and only published study in Indian children [5].
The elemental feeds were found to be clinically equivalent or even superior over steroids in adults with relapses of CD [6]. The efficacy of EEN using a mixed formulation through a nasogastric tube infused by a peristaltic pump was found effective and safe in inducing remission in active CD in children [7]. There are global differences in duration of EEN therapy (6 weeks vs. 8 weeks), type of EEN formulae and methods of re-introducing feeds for pediatric CD [8]. The nutritional and overall outcomes of children with CD in multiple studies were not different and comparable irrespective of the type of feeds used i.e. Polymeric, Semi-elemental, or Elemental [9,10]. Multiple studies over the past decades have shown that the rate of clinical remission did not differ significantly when using amino acid formula or peptide-based formulae [11]. The polymeric formulae group was found to have a higher weight gain in comparison with elemental formulae [12]. The efficacy of polymeric formula vis-a-vis corticosteroids on mucosal healing and clinical variables showed no significant difference in patients getting into remission. However, a significant number of patients achieved mucosal healing with polymeric formulae head-on with corticosteroids which of clinical significance, as PF has no systemic side effects [13]. There is a large variability in the clinical practice guidelines and formulation used in EEN therapy
Polymeric formulas contain whole protein, complex carbohydrates, and long-chain triglycerides. The nutrients in polymeric formulas are intact. Polymeric formulae are less expensive than elemental or semi-elemental formulae. Semi elemental formulae are partially pre-digested. They contain amino acids of varying lengths, simple carbohydrates, and MCTs. The nitrogen source of semi-elemental formulae is a protein that has been hydrolyzed into oligopeptides of varying length, dipeptides, and tripeptides [14,15].
Micronutrient deficiencies are also seen in children with CD. Selenium is an essential micronutrient that is uniquely incorporated into a variety of selenoproteins to impart its beneficial functions. Even though the pathophysiology of IBD is multifactorial in origin, dietary selenium (and selenoprotein) deficiency exacerbates experimental colitis by affecting various signaling pathways involved in inflammation and oxidative stress as well as by altering the gut microbiota. The form of selenium and the duration of supplemental therapy may be important for imparting the beneficial effects of selenium [16]. Selenium nanoparticle (Se NPs) has a role in the management of oxidative stress. They can effectively scavenge different free radicals. The protective effect of Se NPs against oxidative stress is associated with alteration of the innate antioxidant status and mitochondrial function [17].
We compared the efficacy of both SEF and PF on the nutritional status in children with active Crohn’s disease. This study shows our experience of treating pediatric CD with EEN and the effect of using SEF vs. PF on the overall nutritional status.
Materials and Methods
Design
This is a prospective observational study of the pediatric patient (age<17 years) who were diagnosed with acute CD (treatment-naive and relapse) and received EEN for induction of remission at Apollo Children’s Hospital Chennai, India, between 2016 and 2019.
The study was approved by the Institutional Review Board and performed following the principles of the Declaration of Helsinki. Written informed consent was obtained from all the participants.
Children with suspected inflammatory bowel disease who underwent investigations and confirmed the diagnosis of CD was recruited in this study. The child’s family was given a choice of treatment of oral steroids or EEN. The family has been educated about both the therapy and given ample time to conclude which treatment modality they wanted to pursue. The cost to be inferred was discussed and patient leaflets were provided for any questions that they may come up with. Once the decision was made by the family, the preferred treatment was commenced. Oral Azathioprine (Thiopurine) was commenced at a dose range of 1 mg/kg to 2.5 mg/kg per day. The dose was determined depending on their Thiopurine Methyltransferase levels.
All the children who opted for EEN were referred to a qualified clinical Dietitian for nutritional assessment and intervention.
Nutritional Assessment
Nutritional status was analyzed concerning age and gender using weight for height, height for age, Body Mass Index (BMI), and BMI for age percentile using the World Health Organization’s (WHO) growth charts. Water low classification was used for the distinction between wasting (low weight-for-height) and stunting (low height-for-age).
Nutrition Intervention
Nutrition intervention was based on the severity of malnutrition as indicated by the assessment and diet recall. Formulae selection for EEN was based on the target calorie requirement. PF was given in 1 kcal/ml dilution. SEF was started with 1 kcal/ml dilution for the first five days and for those who were not able to meet the target calories, dilution was changed to 1.5 kcal/ml. A customized nutrition care plan and personalized dietary counseling were provided to the child and family.
EEN was commenced on day 1 of the diagnosis. The child’s EEN intake and nutrient requirement were evaluated by a Clinical Dietitian. The energy and volume were increased over 5-7 days depending on the feed tolerance. The nutritional target was to achieve 90%-100% of the Recommended Dietary Allowance (RDA) [18]. The daily nutritive allowances were adjusted according to the individual needs. The physical activity level was observed to adjust the requirements.
Children were allowed to drink water, a can (300 ml) of the fizzy drink of their choice, and a handful of hard-boiled candies daily to keep them motivated during the period of EEN therapy. In our experience, this also helped to avoid dropouts and ensure compliance.
Patients were seen by the dietician and the clinician daily as an outpatient, during the first week (minimum 5 visits), to ensure compliance to the therapy and to monitor any side effects. Also, a weekly contact (telemedicine/phone) with the dietician and the clinician was provided to all patients during the entire course of therapy in the following 8-10weeks. Parents were educated to note the amount of volume consumed by the child daily. At the end of the EEN therapy, a soft consistency diet was introduced slowly with reductions in the formulae feeds on an individual basis.
Laboratory Determinants
Laboratory blood investigation included complete blood count, serum C-reactive protein, serum albumin, and erythrocyte sedimentation rate.
Upper gastrointestinal endoscopy and colonoscopy were performed in all patients before the commencement of EEN therapy. The disease activity was measured using Pediatric Crohn’s Disease Activity Index (PCDAI). PCDAI was calculated using the Cincinnati online calculator [19]. The responsiveness of the PCDAI to improvement in patient clinical status was assessed by comparing data at diagnosis to scores obtained at the end of the EEN therapy. EEN therapy was considered successful if clinical remission was achieved as defined by the PCDAI score, improved nutritional status, and subjective well-being of the child at the end of EEN therapy.
Statistical Analysis
All collected data were transcribed into a Microsoft Excel database and statistically analyzed using Statistical Package for Social Science (SPSS) software version 20.0. Descriptive Data were expressed numerically and as percentiles for categorized data, mean, and SD for parametric diet.
Results
Thirty-eight (n=38) children with confirmed CD were included in the study. 52.6% of children were treated with SEF and 47.4% children with PF orally. The mean age of group SEF and PF were 9.05 ± 3.84, 10.61 ± 3.97 (range 2-16 years) with mean BMI of 14.68 ± 2.37, 14.26 ± 2.73 respectively. A mean increase of 4.46 ± 2.14 kg weight was noted in the SEF group and 4.89 ± 3.0 kg weight was noted in the PF group (p<0.000) (Table 1).
| Anthropometric measurements (pre and post-EEN) | ||||
|---|---|---|---|---|
| Variable | Pre EEN | Post EEN | ||
| SEF | PF | SEF | PF | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Weight (kg) | 27 ± 10.83 | 28.65 ± 10.82 | 31.46 ± 11.64 | 33.54 ± 10.44 |
| Height (cm) | 132.85 ± 19.26 | 139.28 ± 16.98 | 133.4 ± 19.24 | 139.42 ± 16.94 |
| BMI (kg/m2) | 14.68 ± 2.37 | 14.26 ± 2.73 | 17.53 ± 2.85 | 16.83 ± 2.24 |
Table 1: Anthropometric measurement in pre and post EEN period (values are expressed as mean ± SD).
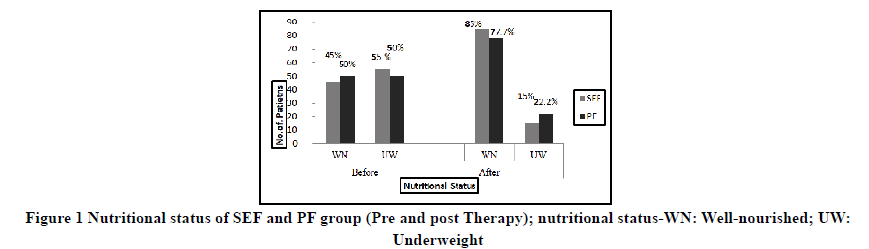
At the onset of therapy, 45% of children were well-nourished in the SEF group and 50% of children were wellnourished in the PF group (Figure 1). The underweight children of both the group were sub-classified based on Waterlow criteria. In both the groups, wasting was most predominant, 45% in SEF and 40% in PF followed by the presence of both wasting and stunting, 10% in SEF, and 11% in PF in the Pre EEN period. The nutritional status improved at the end of EEN therapy. The SEF group had 85% children as well-nourished and 77.7% well-nourished in the PF group.
EEN was given for 8 weeks in both groups. There was a significant improvement in calorie intake in both the group after EEN therapy. PF group calorie intake increased from 1786 ± 477.06 to 1963 ± 506.08 and SEF group increased 1615 ± 471.02 to 1939 ± 593.4. In SEF, 60% of children were given 1.5 kcal/ml dilutions after the first five days they were not able to take the prescribed volume. In the SEF group 93.29% target calorie requirement was met and in the PF group, 90.49% target calorie was met during the EEN therapy (Table 2).
| SEF (Mean ± SD) | PF (Mean ± SD) | |
|---|---|---|
| Home Diet recall | 1615 ± 471.02 | 1786 ± 477.06 |
| Prescribed calorie | 2166.4 ± 624.37 | 2211 ± 549.32 |
| Total energy intake | 1939 ± 593.44 | 1963 ± 506.08 |
Table 2: Nutritional Information of SEF and PF group.
Tolerability and Adverse Effect
All the 38 patients (100%), from both groups, completed 8 weeks of EEN. None of the patients were intolerant to EEN and none had to worsen of disease activity or needed emergency surgical intervention. There were no withdrawals due to adverse events though mild nausea, flatulence, and abdominal bloating were complained by all the patients. However, these were transient, observed in the first 1 week of EEN, and improved subsequently. No serious adverse events were noted.
PCDAI in Treatment Groups
In both the groups there was a significant reduction in the PCDAI at the time of completion of EEN therapy (p<0.000) (Table 3). In our study, a decrease of 11-14 points following therapeutic intervention reflected a clinically significant response.
| PCDAI | Pre EEN Mean ± SD | Post EEN Mean ± SD |
|---|---|---|
| SEF | 37.50 ± 10.97 | 24.50 ± 9.72 |
| PF | 35.78 ± 10.94 | 24 ± 9.37 |
Table 3: PCDAI score (pre and post therapy).
Relapse
Despite the use of azathioprine maintenance therapy, the likelihood of relapsing disease within the first year after EEN treatment was noted in 1 patient each in both groups. Changes in the nutritional status and clinical parameters were used as an endpoint in this study
Discussion
In both the group, one child had a relapse within one year of completion of the primary course of EEN and the child responded well on repeating the EEN. No systemic side effects due to EEN were seen in both groups during the study period. PCDAI differences were seen in all patients and an improvement was noted in both groups.
Polymeric formulae are as effective as elemental in inducing disease remission [20-21]. They are also cost-effective and more palatable. It is often assumed that most, if not all, patients with GI problems have varying levels of malabsorption and/or mal-digestion and would therefore benefit from elemental or semi-elemental formulas.
The difference between PF and SEF is that the protein fraction in the PF is whole vis-a-vis SEF the protein is in peptide form. A clear improvement in nutritional status was obtained on the two groups, without any difference between the two formulae, indicating that irrespective of the form of protein that is given for the child with CD the difference is not seen clinically or statistically.
In our center’s experience compliance in both the SEF and PF group has been equivocal. In our experience to meet the calorific demands and needs of the individual, the volume of the feeds was customized. The concentration of feeds may be done if calorific requirements are to be met within a specific/lesser volume. This aids compliance if the prescribed volume is acceptable to the child. The standard concentration of PF formulae is 1 kcal/ml but in SEF, concentrations up to 1.5 kcal/ml may be tolerated.
In all the patients, weight for height improved significantly between the commencement and completion with EEN. Significant weight gain was noted in both PF and SEF groups at the end of therapy. EEN using either formula was effective in inducing clinical remission in active pediatric Crohn’s disease. The type of enteral feeds (peptide or intact protein) used in EEN therapy for CD is not relevant to their therapeutic efficacy.
Cost analysis was made by presumptive calculation that for an 8-year old, weighing 25 kg requiring 1600 kcal/day for 8 weeks will be as follows-Polymeric feed-1 kcal/ml would cost 352 US$, on SEF at 1 kcal/ml-690 US$ and SEF at 1.5 kcal/ml would cost 920 US$ for the entire course of treatment for 8 weeks.
We acknowledge the limitations of our study. It is an observational, single-center study with a small number of patients and lacks gut microbiome analysis. Also, we did not examine endoscopic findings at the end of the therapy. More data from prospective trials with a larger number of patients is required.
Conclusion
This is the first prospective pediatric study to compare PF and SEF in the treatment of active Crohn’s disease in India. The results indicate that polymeric formulae are as effective as Semi elemental formulae in inducing short-term remission in acute pediatric Crohn’s disease.
Taste and compliance by the patient for the first five days were taken into consideration for tolerance. None of the patients had intolerance and none had worsening of disease activity.
Nutritional target requirements can be met with SEF with increased concentration of feeds. The particular disadvantages of SEF as reported by the patients have been palatability and the cost when compared to PF.
We aim to further research and collate data in the above cohort and look at long-term follow-up and outcomes in these children with CD. We also plan to do a multi-center clinical trial in children with CD to understand the benefits of EEN and evaluate the efficacy of the same in children.
The polymeric formulae used in our study were Groviva® from Signutra Inc, Pediasure® from Abbott Nutrition, Pediagold® from Hexagon Nutrition.
The Semi elemental formulae used were Peptamen Junior®, Nestle’ Switzerland, and Pediagoldplus®, Hexagon nutrition.
Declaration
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Crohn, Burrill B., Leon Ginzburg, and Gordon D. Oppenheimer. "Regional ileitis: A pathologic and clinical entity."Journal of the American Medical Association,Vol. 99, No. 16, 1932, pp. 1323-29.
- Foss, Harold L., and William T. Barnes. "Segmental ileitis."Annals of Surgery,Vol. 133, No. 5, 1951, pp. 651-63.
- Cooke, W. T. "Nutritional and metabolic factors in the aetiology and treatment of regional ileitis: Hunterian lecture delivered at the Royal College of Surgeons of England on 28th April 1955."Annals of the Royal College of Surgeons of England,Vol. 17, No. 3, 1955, pp. 137-58.
- Voitk, Andrus J., et al. "Experience with elemental diet in the treatment of inflammatory bowel disease: Is this primary therapy?"Archives of Surgery,Vol. 107, No. 2, 1973, pp. 329-33.
- Sreedharan, L. "Management of pediatric Crohn’s disease using exclusive enteral nutrition the Indian subcontinent." Conference Proceeding: Journal of Vaccines and Vaccination, Vol. 9, 2018, p. 23.
- O'Morain, C. "Elemental diets and Crohn's disease."Acta Gastro-Enterologica Belgica,Vol. 50, No. 5, 1987, pp. 574-78.
- Navarro, J., et al. "Prolonged constant rate elemental enteral nutrition in Crohn's disease."Journal of Pediatric Gastroenterology and Nutrition,Vol. 1, No. 4, 1982, pp. 541-46.
- Whitten, Kylie E., et al. "International survey of enteral nutrition protocols used in children with Crohn's disease."Journal of Digestive Diseases,Vol. 13, No. 2, 2012, pp. 107-12.
- Cosnes, J., et al. "Crohn's disease and enteral feeding: comparative nutritional efficacy of elemental and polymeric nutritive mixtures."Annales de Gastroenterologie et d'Hepatologie, Vol. 24, No. 5, 1988, pp. 233-40.
- Rigaud, D., et al. "Controlled trial comparing two types of enteral nutrition in treatment of active Crohn's disease: Elemental versus polymeric diet."Gut,Vol. 32, No. 12, 1991, pp. 1492-97.
- Royall, D., et al. "Comparison of amino acid v peptide based enteral diets in active Crohn's disease: Clinical and nutritional outcome."Gut,Vol. 35, No. 6, 1994, pp. 783-87.
- Ludvigsson, J. F., et al. "Elemental versus polymeric enteral nutrition in paediatric Crohn's disease: A multicentre randomized controlled trial."Acta Paediatrica,Vol. 93, No. 3, 2004, pp. 327-35.
- Borrelli, Osvaldo, et al. "Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: A randomized controlled open-label trial."Clinical Gastroenterology and Hepatology,Vol. 4, No. 6, 2006, pp. 744-53.
- Farrell, James J. "Digestion and absorption of nutrients and vitamins."Sleisenger and Fordtran's Gastrointestinal and Liver Disease. WB Saunders, 2010, pp. 1695-733.
- Makola, Diklar. "Elemental and semi-elemental formulas: Are they superior to polymeric formulas?"Practical Gastroenterology,Vol. 29, No. 12, 2005, pp. 59-72.
- Hiller, Franziska, et al. "Differential acute effects of selenomethionine and sodium selenite on the severity of colitis."Nutrients,Vol. 7, No. 4, 2015, pp. 2687-706.
- Amani, Hamed, et al. "Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries."Journal of Materials Chemistry B,Vol. 5, No. 48, 2017, pp. 9452-76.
- Gopalan, Coluthur, B. V. Rama Sastri, and S. C. Balasubramanian. "Nutritive value of Indian foods." National Institute of Nutrition, Indian Council of Medical Research, 1971.
- Hyams, Jeffrey S., et al. "Development and validation of a pediatric Crohn's disease activity index."Journal of Pediatric Gastroenterology and Nutrition,Vol. 12, No. 4, 1991, pp. 439-47.
- Verma, S., et al. "Polymeric versus elemental diet as primary treatment in active Crohn’s disease: A randomized, double-blind trial."The American Journal of Gastroenterology,Vol. 95, No. 3, 2000, pp. 735-39.
- Sakurai, Toshihiro, et al. "Shortâ?term efficacy of enteral nutrition in the treatment of active Crohn's disease: A randomized, controlled trial comparing nutrient formulas."Journal of Parenteral and Enteral Nutrition,Vol. 26, No. 2, 2002, pp. 98-103.