Research - International Journal of Medical Research & Health Sciences ( 2023) Volume 12, Issue 2
Induction of Bystander Response in Lung Cancer Cells under Photon Beam Irradiation using Bismuth Sulfide Nanoparticles
Leila Nasehi1,2, Hamed Rezaeejam3, Hossein Danafar4, Parvin Mirzaghavami3, Ali Mohammadi4, Mahda Delshad2 and Massoud Vosough5,6*2Department of Medical Laboratory, School paramedical Sciences, Zanjan University of Medical Sciences, Zanjan, Iran
3Department of Radiology, School paramedical Sciences, Zanjan University of Medical Sciences, Zanjan, Iran
4Zanjan Pharmaceutical Biotechnology Research center, Zanjan University of Medical Sciences, Zanjan, Iran
5Department of Regenerative Medicine, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran
6Experimental Cancer Medicine, Institution for Laboratory Medicine, Karolinska Institute, Stockholm, Sweden
Massoud Vosough, Experimental Cancer Medicine, Institution for Laboratory Medicine, Karolinska Institute, Stockholm, Sweden, Email: masvos@Royaninstitute.org
Received: 20-Jan-2023, Manuscript No. ijmrhs-23-87506; Editor assigned: 23-Jan-2023, Pre QC No. ijmrhs-23-87506(PQ); Reviewed: 26-Jan-2023, QC No. ijmrhs-23-87506(Q); Revised: 05-Feb-2023, Manuscript No. ijmrhs-23-87506(R); Published: 28-Feb-2023
Abstract
Background: The use of nanomaterial-based radiosensitizers to improve the therapeutic ratio has gained attraction in radiotherapy. Increased radiotoxicity applied to the tumor region may result in an adverse impact on the unexposed normal cells to the radiation, a phenomenon known as Radiation-Induced Bystander Effect (RIBE). Objective: This study aimed to investigate the effect of Bi2S3@BSA Nanoparticles (NPs) as radiosensitizers on the enhancement of bystander response in non-irradiated cells. Methods: Lung carcinoma epithelial cells were exposed to 6 MV x-ray photons at different doses of 2 and 8 Gy, with and without Bi2S3@BSA NPs. The Irradiated Cells Conditioned Medium (ICCM) was collected and incubated with MCR-5 human fetal lung fibroblasts. Results: This study showed that ICCM collected from 2-Gy-irradiated A549 cells in the presence of Bi2S3@BSA NPs reduced the cell viability of MCR-5 Bystander cells more than ICCM collected from irradiated cells without NPs (p<0.05), whereas such a difference was not observed after 8-Gy radiation. The mRNA expression of the BAX and XPA genes, as well as the cell death rate in MCR-5 bystander cells, revealed that the Bi2S3@BSA NPs significantly improved bystander response at 2 Gy (p<0.05), but the efficacy was not statistically significant after 8 Gy Irradiation. Conclusions: The results indicated that the presence of NPs did not affect bystander response enhancement at higher concentrations. These findings highlighted the potential use of radiation-enhancing agents and their benefits in radiotherapy techniques with high doses per fraction.
Keywords
Bismuth nanoparticles, Lung cancer, Radiation therapy, Bystander effect
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of death worldwide, accounting for 1.6 million deaths each year. Ionizing radiation therapy is a common treatment for lung cancer at all stages [1]. One of the major concerns related to radiation therapy is the side effects on normal tissues. To address this issue, advanced technologies such as Intensity-Modulated Radiotherapy (IMRT) have been developed, which allowed high doses to be delivered to irregularly shaped target volumes while minimizing doses to nearby vulnerable normal structures. Despite the high dose conformity provided by advanced radiation therapy modalities, complete sparing of normal tissue would be impossible, and the volume of normal tissue exposed to low-dose radiation would be more in conventional radiotherapy due to the use of multiple field sources [2]. Another strategy for reducing unwanted adverse impacts on normal tissue is to use radio-sensitizers, which increase radiation efficiency only in the tumor site. Because NPs preferentially accumulate in tumor regions as a result of Enhanced Permeability and Retention (EPR), they can be used as a radiosensitizer [3]. Active targeting of NPs can improve their absorbance by cancer cells in addition to the EPR effect [4-6]. In this regard, the use of metal-based nanomaterials as radio-sensitizers has piqued the interest of many researchers [7-10]. Bismuth-based NPs, such as Bismuth Sulfide (Bi2S3), have been proposed as a suitable candidates for use in conjunction with ionizing radiation treatment. This choice was made for several reasons, including its high photoelectric absorption coefficient due to its high atomic number, low toxicity, and low cost [11-13]. Furthermore, bismuth-containing compounds are widely used in current medicine, e.g., gastritis due to Helicobacter pylori infection, demonstrating their biocompatibility [14].
Furthermore, it is well established that unirradiated cells outside the treatment field would show some characteristics of irradiated cells such as altered apoptosis, increased mutation, and reduced clonogenicity. This is known as the Radiation-Induced Bystander Effect (RIBE) [15,16]. Bystander signal transmission appears to occur via cell-to-cell contact or via released soluble factors in the culture medium. Many studies have been conducted to investigate the relationship between bystander signals and oxidative stress. Nitric Oxide (NO), Transforming Growth Factor-Beta-1 (TGF-1), Interleukin (IL-1), (IL-2), (IL-8), and Tumor Necrosis Factor (TNF) have all been identified as bystander signaling factors [17,18].
Although it has been demonstrated that NPs alter ROS production, cytokine profile, gene expression pattern, and thus bystander signaling a few studies have investigated the impact of radiation-enhancing agents to induce bystander responses [19-21].
This study aimed to examine the effects of Bismuth Sulfide NPs on the induction of bystander responses to MCR-5, human fetal lung fibroblast cells, as well as its Radiation-Enhancing Efficiency in A549, lung carcinoma epithelial cells, and how this affects therapeutic ratio.
Methods
Cell Culture
In this study, MCR-5, human fetal lung fibroblast cells, and A549, lung carcinoma epithelial cells, were used as bystander and target cells, respectively. Both cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% FBS, Penicillin (100 U/mL), and Streptomycin (1%) (100 mg/mL) at 37°C in a humidified incubator with 5% carbon dioxide.
Synthesis and Characterization of NPs
Bi2S3 coated with bovine Serum Albumin (Bi2S3@BSA) was synthesized and characterized using the processes described in a previous study [22]. To reduce NP aggregation and agglomeration, they were suspended in the medium and vortexed for 30 seconds before use.
Irradiation of Cells
The A549 cells were seeded at a density of 2×105 cells per flask (25 cm2 ) and used as target cells. The cells were incubated for 24 hours with 40 μg mL-1 of Bi2S3@BSA NPs. The confluency of cells was about 50% at the time of irradiation. This cell density primarily allows cell-cell contact only through released soluble factors in the medium. The cells in flasks were exposed to 2 Gy and 8 Gy of a 6 MV photon beam generated by a linear accelerator (Siemens, Germany) at Valiasr Hospital under the following conditions beam field of 1.01 m and distance of 100 cm between the radiation source and flask bottom.
Medium Transfer
The flasks were incubated at 37°C for 4 hours following the irradiation. The medium was then transferred using the technique developed by Mothersill and Seymour [23. To avoid irradiated cells entering the transferred medium, the Irradiated-Cell Conditioned Medium (ICCM) was extracted from the flasks and filtered through 0.22 m Polyethersulfone (PES) membrane filters. For further experiments, the ICCM was transferred into MCR-5 cells.
Viability Assay
The MTT viability assay was used to assess the effect of Bi2S3@BSA NPs on radiation-induced bystander response in MCR-5 cells, as well as their cytotoxicity and radiosensitizing efficacy in A549 target cells. The A549 target cells were cultured in a 96-well plate at a density of 3×103 cells per well in a medium to investigate the radiosensitization of NPs. The cells were incubated with different concentrations (5 μg/mL,10 μg/mL,20 μg/mL,40 μg/mL, and 80 μg/mL) of Bi2S3@BSA NPs for 24 hours before being exposed to radiation. MCR-5 cells were seeded in a 96-well plate at a density of 5×103 in ICCM extracted from treated flasks of the target cells to assess the effect of the Bi2S3@BSA NPs on bystander response. The plate was incubated for 24 hours. Following that, the cells were washed and treated with 5 mg/ml MTT before incubation at 37°C for 4 hours in the dark. The MTT solution was then discarded, and 100 µl of Dimethyl Sulfoxide (DMSO) was added to dissolve the formazan crystals. After 15 minutes, the absorbance was measured at 570 nm using a microplate reader (BioTek, Winooski, VT). To determine cell viability, the absorbance values were normalized to those of control cells.
Gene Expression Study Using Quantitative Real-time PCR (qPCR)
After treatment, the cells were harvested with trypsin and washed with PBS. Then Trizol reagent was used to extract the total RNA from all samples according to the manufacturer's instructions (Invitrogen Life Technologies Co., Waltham, MA). The concentration and purity of extracted RNA were determined using a NanoDrop OneSpectrophotometer (Thermo Scientific, USA). Then, according to the instructions of the cDNA Synthesis kit (Fermentas), 2 g of total RNA from each sample was used to synthesize complementary DNA (cDNA). The cDNA was then subjected to real-time PCR to investigate the gene expression of BAX and XPA, which are key players in apoptosis and DNA repair pathways, respectively. The XPA gene has been linked to Double-Strand Break (DSB) repair via the Nucleotide Excision Repair (NER) pathway [24].
As a housekeeping gene, β-ACTIN was used. The reaction mixture was prepared according to the instructions of PCR Master Mix Green (Ampliqon, Denmark) using a specific primer sequence and transferred to real-time PCR Step One Plus (Applied Biosystems, USA). The reaction conditions were as follows: initial denaturation at 95°C for 15 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds, and annealing/extension at 60°C for 60 seconds. The expression of the BAX and XPA genes was calculated using 2-ΔΔCT .
Flow Cytometry Analysis of Cell Apoptosis
The Annexin V-FITC Apoptosis Detection Kit (eBioscience) was used to examine the rate of apoptosis and necrosis in the A549 target and MCR-5 bystander cells, according to the manufacturer's instructions. Cells were detached, counted, and washed with cold PBS before being suspended in 100 µL of binding buffer 1X. The cells were then incubated for 15 minutes at room temperature in dark with 5 µL of Annexin V-FITC. The cells were then washed and resuspended in 400 µL of binding buffer 1X. Following that, the fluorescent intensity of each sample was measured after adding 5 µL PI to generate an FL-1 vs. FL-2 plot using the BD FACS Calibur (BD, San Jose, CA, USA).
Data Analysis
The data were analyzed using one-way analysis of variance (ANOVA) in GraphPad prism. The cut-off level of significance was considered p<0.05 (n=3). The results were represented as Mean ± SD.
Results
MTT Viability Assay
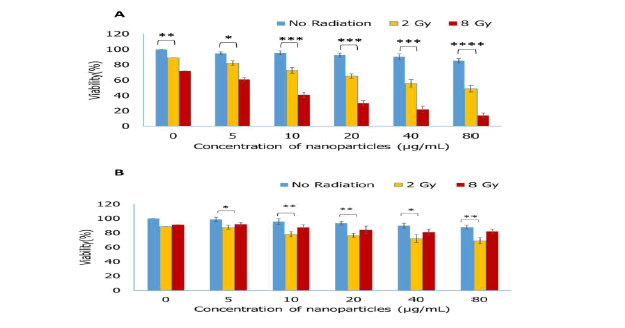
MTT assay was employed to assess the effect of Bi2S3@BSA NPs on the viability of A549 target cells and MCR-5 bystander cells. The viability of nanoparticle-incubated A549 cells at both doses of 2 Gy and 8 Gy was significantly lower than that of cells that were exposed to radiation alone at all used concentrations of Bi2S3@BSA NPs. This effect was enhanced as the concentration of NPs increased. Additionally, Bi2S3@BSA NPs showed higher radiosensitizing enhancement for the 8 Gy dose of radiation.
The viability of MCR-5 bystander cells that received ICCM from 2 Gy irradiated A549 cells incubated with Bi2S3@BSA NPs at concentrations more than 10 µg/L was significantly lower than that of groups that received ICCM from A549 cells exposed only to radiation (p<0.05). However, at a radiation dose of 8 Gy, this difference appeared to be insignificant at all concentrations. This finding indicates that Bi2S3@BSA NPs had no efficacy in improving bystander responses in MCR-5 at a higher dose of 8 Gy (Figure 1).
Figure 1. Cell Viability, the effect of different concentrations of Bi2S3@BSA NPs with and without radiation on the viability of (A) A549 target cells and (B) MCR-5 bystander cells was assessed by MTT assay. The signs of *, **, ***, and **** are represented for p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001respectively
Gene Expression Analysis (qPCR)
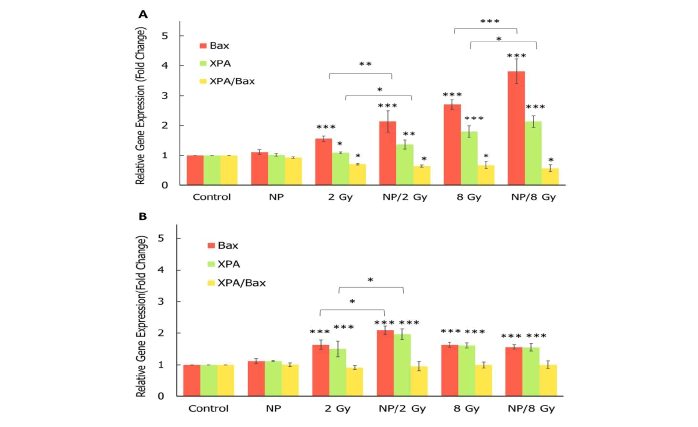
Molecular studies were conducted to investigate the effect of Bi2S3@BSA NPs on A549 target cells and MCR-5 bystander cells as radio-sensitizers and bystander response inducers, respectively. At both doses of 2 Gy and 8 Gy, significant up-regulation of BAX, a proapoptotic gene, and XPA, a DNA repair-involved gene, was observed in A549 target cells (p<0.001). Furthermore, expressions of both the BAX and XPA genes increased with irradiation (at both 2 Gy and 8 Gy doses) in the presence of Bi2S3@BSA NPs (p<0.05). This demonstrates the nanoparticle's efficient radiosensitizing property.
At the radiation dose of 2 Gy, the expression of the BAX and XPA genes was upregulated in MCR-5 bystander cells, and the upregulation was even more when radiation and NPs were combined. However, at radiation doses of 8 Gy, these upregulations decreased and were less than upregulations at 2 Gy. Surprisingly, no significant difference in BAX and XPA gene expression was observed between 8 Gy/NPs treatment and 8 Gy alone. Furthermore, the XPA/BAX ratio was determined in both target and bystander cells to compare the roles of repair and apoptotic pathways. In MCR-5 bystander cells, there was no significant difference in the XPA/BAX ratio between the groups, whereas this ratio decreased in A549 target cells as treatment was intensified (Figure 2).
Analysis of Induced Apoptosis and Necrosis
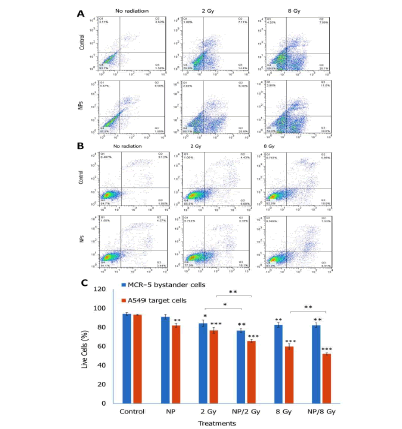
The apoptotic and necrotic rates in the A549 target and MCR-5 bystander cells were determined using flow cytometry and Annexin V-FITC/PI staining (Figure 3). Flow cytometry analysis revealed that the mean percentage of overall cell death (early apoptosis+late apoptosis+necrosis) caused by 2 Gy and 8 Gy of 6 MV x-ray photons was 23.2% and 41.8%, respectively, 24 hours after irradiation in A549 target cells. As irradiation was applied in the presence of Bi2S3@BSA NPs, these death rates increased to 34.2% and 47.6%, respectively. These differences in cell death with and without Bi2S3@BSA NPs demonstrate that the aforementioned NPs efficiently played the role of a radio-sensitizing agent at both radiation doses. The amount of cell death in MCR-5 cells incubated with ICCM extracted from treated A549 cells was measured to assess the bystander response-inducing ability of Bi2S3@BSA NPs. The percentage of live cells in MCR-5 cells incubated with ICCM from 2 Gy and 8 Gy radiated cells was 84.13.62% and 82.82.54%, respectively (Figure 3). When combined with Bi2S3@BSA NPs, these rates increased to 76.52.2% and 82.12.48%, respectively. This supports the efficacy of Bi2S3@BSA NPs as bystander-inducing enhancers only at 2 Gy (p<0.01).
Discussion
The Radiation-Induced Bystander Effect (RIBE) is a phenomenon in which unirradiated cells outside of the radiation field appear to be affected by radiation by receiving signals from nearby irradiated cells [25]. The use of NPs as radiation enhancers has been shown to protect normal tissue from the effects of direct radiation [26,27]. Nonetheless, a few studies have dealt with the effects of NPs on normal tissue via bystander signaling. Evaluating the role of NPs as bystander response inducers in normal cells could be critical in clinical applications. If NPs delivered to cancer cells increase bystander responses in surrounding normal cells, their efficacy for increasing the therapeutic ratio will be diminished or even neutralized.
This study investigated the effect of Bi2S3@BSA NPs as an inducer of bystander responses. It also aimed to determine whether the ability of these NPs to enhance bystander signaling is comparable to their ability to radiosensitize directly irradiated cells. This comparison helped to estimate the effects of NPs on the therapeutic ratio. Although designing and developing new radio sensitizers have received great attention in the last decade, a limited number of studies investigated the effects of radiosensitizers on bystander responses [20,21]. The findings of a study showed that Bi2O3 NPs could not improve bystander response in MCF-7 cancer cells or human fetal osteoblast (hFOB 1.19) as normal cells at doses less than 10 Gy [20]. Another study reported that glucose-coated gold NPs (Glu-GNPs) increased bystander response in QUDB cells at a dose of 2 Gy of 100 kVp X-rays, but did not affect RIBE in MCF-7 cells [21]. Furthermore, cell type may be one of the factors influencing the efficacy of NPs on bystander response. The gene expression experiments in this study revealed that the BAX and XPA genes were upregulated at a radiation dose of 2 Gy and that this upregulation was enhanced when radiation and NPs were combined. However, these increases were lower at radiation doses of 8 Gy compared to upregulations at 2 Gy. However, there was no statistically significant difference in BAX and XPA gene expression between 8 Gy radiation combined with NPs and the same radiation alone. This pattern of variations suggests that bystander signaling may be more effective at lower ionizing radiation doses. The lack of difference between 8 Gy and 8 Gy /NPs demonstrated that bystander signaling would not increase even with NPs. These findings could imply that bystander signaling can become saturated in more intense treatments. Consistent with the findings of this study, Researcher reported downregulation of BAX gene expression at 6 Gy and 8 Gy of gamma rays, as well as a significant reduction of the XPA repair gene at 8 Gy compared to lower doses in QU-DB bystander cells [28]. Researcher discovered that transferring medium extracted from 0.5 Gy and 5 Gy irradiated cells to bystander cells reduces bystander cell survival fraction. Bystander response was eliminated in both electron beam and gamma irradiation as the dose reached 10 Gy. This finding leads to the hypothesis that higher doses of radiation cause negative feedback in bystander cells. This negative feedback appears to be caused by increased TGF-β-related signaling from target cells, which reduces bystander response [29]. This hypothesis was also tested by diluting ICCM extracted from QU-DB cells that had been irradiated with 6 Gy and 8 Gy. It was discovered that 8% diluted medium from 6 Gy and 6% diluted medium from 8 Gy resulted in the greatest number of micronucleated cells [30]. Consistent with the above-mentioned study, this study demonstrated that, in bystander cells, the application of higher doses of 8 Gy resulted in increased viability, decreased overall cell death, and decreased upregulation of the BAX proapoptotic gene and the XPA repair gene. Furthermore, the presence of NPs did not affect bystander responses at an 8 Gy radiation dose. The findings hence agreed with the previously mentioned negative feedback. Bystander cells also have enhanced DNA repair mechanisms, which help them avoid cell death and, as a result, promote radioresistance. In this regard, Iyer et al. found that human fibroblast cells previously exposed to conditioned media from irradiated fibroblasts exhibited increased radioresistance due to an increase in AP-Endonuclease, a DNA base excision repair enzyme [31]. The main factor in the development of carcinogenesis is the promotion of cell death evasion. As a result, bystander signals may cause tumor induction in other healthy tissues [32].
Our findings suggested that the use of NPs as a radiation enhancer agent in some radiotherapy techniques, such as IMRT, stereotactic radiosurgery, brachytherapy, intraoperative radiotherapy, and hypofractionated treatments, which apply a high dose per fraction, seems to be more beneficial than using them in techniques based on a low-dose per fraction. This is due to the adverse effects of bystanders on normal tissues.
Conclusion
This study assessed the effect of nano-radiosensitizer on the outcomes of ionizing radiation treatments. Based on our findings, the application of NPs did not increase bystander signals at higher doses of radiation. This finding may emphasize the safe and beneficial use of these NPs in radiotherapy techniques with higher doses per fraction.
Declarations
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by the Zanjan University of Medical Sciences (Grant no: A-10-1357-4 and A-11-1357-3).
Author Contribution
Leila Nasehi Resources, Conceptualization, Formal analysis, Investigation, Writing - Original Draft. Hamed Rezeejam Formal analysis, Investigation, Writing-Original Draft, Writing-Review & Editing. Hossein Danafar Methodology, Investigation. Parvin Mirzaghavami Writing-Original Draft, Methodology, Investigation. Ali Mohammadi Methodology, Investigation. Mahda Delshad Methodology. Massoud Vosough Conceptualization, Methodology, Software, Formal analysis, Writing-Review & Editing.
References
- Brown, Sean, et al. "The evolving role of radiotherapy in non-small cell lung cancer." The British journal of radiology, Vol. 92, No. 1104, 2019.
Google Scholar Crossref - Prise, Kevin M., and Joe M. O'sullivan. "Radiation-induced bystander signalling in cancer therapy." Nature Reviews Cancer, Vol. 9, No. 5, 2009, pp. 351-60.
Google Scholar Crossref - Stylianopoulos, Triantafyllos. "EPR-effect: utilizing size-dependent nanoparticle delivery to solid tumors." Therapeutic delivery, Vol. 4, No. 4, 2013, pp. 421-23.
Google Scholar Crossref - Yoo, Jihye, et al. "Active targeting strategies using biological ligands for nanoparticle drug delivery systems." Cancers, Vol. 11, No. 5, 2019, p. 640.
Google Scholar Crossref - Mirzaghavami, Parvin S., et al. "Folic acid-conjugated magnetic triblock copolymer nanoparticles for dual targeted delivery of 5-fluorouracil to colon cancer cells." Cancer Nanotechnology, Vol. 13, No. 1, 2022, p. 12.
Google Scholar - Nosrati, Hamed, et al. "Folic acid conjugated bovine serum albumin: An efficient smart and tumor targeted biomacromolecule for inhibition folate receptor positive cancer cells." International journal of biological macromolecules, Vol. 117, 2018, pp. 1125-32.
Google Scholar Crossref - Guerreiro, Alexandra, et al. "A comparison of the radiosensitisation ability of 22 different element metal oxide nanoparticles using clinical megavoltage X-rays." Cancer Nanotechnology, Vol. 10, No. 1, 2019, pp. 1-20.
Google Scholar - Sherstiuk, Anastasiia A., et al. "Hafnium Oxide-Based Nanoplatform for Combined Chemoradiotherapy." ACS Biomaterials Science & Engineering, Vol. 7, No. 12, 2021, pp. 5633-41.
Google Scholar Crossref - Mirzaghavami, Parvin S., et al. "Radio-sensitivity enhancement in HT29 cells through magnetic hyperthermia in combination with targeted nano-carrier of 5-Flourouracil." Materials Science and Engineering, Vol. 124, 2021.
Google Scholar Crossref - Pandey, Arvind, et al. "Gold Nanoparticles Radio-Sensitize and Reduce Cell Survival in Lewis Lung Carcinoma." Nanomaterials, Vol. 10, No. 9, 2020, p. 1717.
Google Scholar Crossref - Nosrati, Hamed, et al. "Tumor targeted albumin coated bismuth sulfide nanoparticles (Bi2S3) as radiosensitizers and carriers of curcumin for enhanced chemoradiation therapy." ACS Biomaterials Science & Engineering, Vol. 5, No. 9, 2019, pp. 4416-24.
Google Scholar Crossref - Wang, Yong, et al. "BSA‐mediated synthesis of bismuth sulfide nanotheranostic agents for tumor multimodal imaging and thermoradiotherapy." Advanced Functional Materials, Vol. 26, No. 29, 2016, pp. 5335-44.
Google Scholar Crossref - Faghfoori, Mohammad H., et al. "Anticancer effect of X-Ray triggered methotrexate conjugated albumin coated bismuth sulfide nanoparticles on SW480 colon cancer cell line." International Journal of Pharmaceutics, Vol. 582, 2020.
Google Scholar Crossref - Griffith, Darren M., et al. "Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles." Chemical Society Reviews, Vol. 50, No. 21, 2021, pp. 12037-69.
Google Scholar - Butterworth, Karl T., et al. "Out-of-field cell survival following exposure to intensity-modulated radiation fields." International Journal of Radiation Oncology* Biology* Physics, Vol. 79, No. 5, 2011, pp. 1516-22.
Google Scholar Crossref - Havaki, Sophia, et al. "The role of oxidative DNA damage in radiation induced bystander effect." Cancer letters, Vol. 356, No. 1, 2015, pp. 43-51.
Google Scholar Crossref - Little, John B. "Cellular radiation effects and the bystander response." Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, Vol. 597, No. 1-2, 2006, pp. 113-18.
Google Scholar Crossref - Yang, Shuning, et al. "Radiation-induced bystander effects in A549 cells exposed to 6 MV X-rays." Cell biochemistry and biophysics, Vol. 72, No. 3, 2015, pp. 877-82.
Google Scholar Crossref - Verma, Neha, and Ashu B. Tiku. "Significance and nature of bystander responses induced by various agents." Mutation Research/Reviews in Mutation Research, Vol. 773, 2017, pp. 104-21.
Google Scholar Crossref - Zainudin, Nur-Hamizah M., et al. "Influence of bismuth oxide nanoparticles on bystander effects in MCF-7 and hFOB 1.19 cells under 10 MV photon beam irradiation." Radiation Physics and Chemistry, Vol. 177, 2020.
Google Scholar Crossref - Rostami, Atefeh, et al. "The effect of glucose-coated gold nanoparticles on radiation bystander effect induced in MCF-7 and QUDB cell lines." Radiation and environmental biophysics, Vol. 55, 2016, pp. 461-66.
Google Scholar Crossref - Nosrati, Hamed, et al. "Complete ablation of tumors using synchronous chemoradiation with bimetallic theranostic nanoparticles." Bioactive materials, Vol. 7, 2022, pp. 74-84.
Google Scholar Crossref - Mothersill, Carmel, and C. B. Seymour. "Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium." Radiation research, Vol. 149, No. 3, 1998, pp. 256-62.
Google Scholar Crossref - Zhang, Ye, et al. "Suppressed expression of non-DSB repair genes inhibits gamma-radiation-induced cytogenetic repair and cell cycle arrest." DNA repair, Vol. 7, No. 11, 2008, pp. 1835-45.
Google Scholar Crossref - Seymour, Colin B., and Carmel Mothersill. "Radiation-induced bystander effects—implications for cancer." Nature Reviews Cancer, Vol. 4, No. 2, 2004, pp. 158-64.
Google Scholar Crossref - Pan, Yue, et al. "Metal-based hybrid nanoparticles as radiosensitizers in cancer therapy." Colloid and Interface Science Communications, Vol. 23, 2018, pp. 45-51.
Google Scholar Crossref - Cui, Lei, et al. "Radiosensitization by gold nanoparticles: Will they ever make it to the clinic?." Radiotherapy and Oncology, Vol. 124, No. 3, 2017, pp. 344-56.
Google Scholar Crossref - Toossi, Mohammad T.B., et al. "Regulation of XPA could play a role in inhibition of radiation-induced bystander effects in QU-DB cells at high doses." Journal of Cancer Research and Therapeutics, Vol. 16, No. 1, 2020, pp. 68-73.
Google Scholar Crossref - Gow, M. D., et al. "Effect of dose rate on the radiation-induced bystander response." Physics in Medicine & Biology, Vol. 53, No. 1, 2007, p. 119.
Google Scholar Crossref - Toossi, Mohammad T.B., et al. "Assessment of the dose-response relationship of radiation-induced bystander effect in two cell lines exposed to high doses of ionizing radiation (6 and 8 Gy)." Cell Journal (Yakhteh), Vol. 19, No. 3, 2017, p. 434.
Google Scholar Crossref - Iyer, Rashi, and Bruce E. Lehnert. "Alpha-particle-induced increases in the radioresistance of normal human bystander cells." Radiation research, Vol. 157, No. 1, 2002, pp. 3-7.
Google Scholar Crossref - Baskar, Rajamanickam. "Emerging role of radiation induced bystander effects: Cell communications and carcinogenesis." Genome integrity, Vol. 1, No. 1, 2010, pp. 1-8.
Google Scholar Crossref