Special Issue - International Journal of Medical Research & Health Sciences ( 2021) Volume 0, Issue 0
Pulmonary vasculopathy: A mechanism for �??silent hypoxemia�?? in patients with covid-19
Mohammed Fawzi Abosamak1, Rajkumar Rajendram2, Mohamed Buheji3*, Mohamed Makhloof Aldahish4, Abdulaziz Ali Alkhereiji5 and Ka Ting Ng62Department of Medicine, King Abdulaziz International Medical Research Center, Ministry of National Guard - Health Affairs, Riyadh, Saudi Arabia
3International Inspiration Economy Project, Bahrain
4Department of Anesthesia and Intensive Care, Faculty of Medicine, Cairo University, Egypt
5Department of Anesthesia, Security Forces Hospital, Riyadh, KSA
6Department of Anesthesia, University of Malay, Kuala Lumpur, Malaysia
Mohamed Buheji, International Inspiration Economy Project, Bahrain, Email: buhejim@gmail.com
Received: 05-Mar-2021 Accepted Date: Mar 22, 2021 ; Published: 30-Mar-2021, DOI: 0
Abstract
Silent or ‘happy’ hypoxemia has been noticed in patients with Coronavirus Disease 2019 (COVID-19). In some of the hypoxemic COVID-19 patients, there was no correlation between the degree of hypoxemia and the severity of parenchymal lung injury on chest imaging. The presence of micro thrombi in the peripheral pulmonary vasculature, endotheliitis, vasculitis and perivascular inflammation was proposed to lead to vascular shunt that causes ventilation/perfusion mismatch. Thus, the occurrence of silent hypoxemia can be explained by the characteristic pulmonary vasculopathy reported in COVID-19 patients. Silent hypoxemia phenomenon should encourage clinicians to routinely use pulse oximetry and use the optimum diagnostic modality for diagnosis of pulmonary vascular micro thrombi. The role of using systemic thrombolytics to treat refractory hypoxemia due to vascular shunt secondary to pulmonary micro thrombi should be investigated to test both the benefits and harms. Future research should study the benefits and the optimum doses of intravenous thrombolytic therapy in severe cases of COVID-19 with silent hypoxemia.
Introduction
COVID-19 disease presentation is highly variable, with some patients having minimal symptoms and others developing severe respiratory failure and Acute Respiratory Distress Syndrome (ARDS). Although some individuals with COVID-19-induced hypoxemia experience dyspnea, defined as breathing discomfort [1], others do not. Those latter patients have been labeled with ‘silent hypoxemia’ [2,3].
The sensation of dyspnea may represent a conscious awareness of the outgoing respiratory motor command, in which areas of the brain that control ventilation sends efferent commands to the ventilatory muscles, and a neurological copy of these commands is sent to the sensory cortex [4]. Therefore, disconnect that can occur between the control of breathing and respiratory sensation, when anticipated responses to stimuli do not occur because of impaired lung or chest wall mechanics [5]. Additionally, cytokines, may independently either trigger or suppress dyspnea, depending on how the specific cytokine interacts with immune cells [6,7] such as the ‘cytokine storm’ typically associated with ARDS [8].
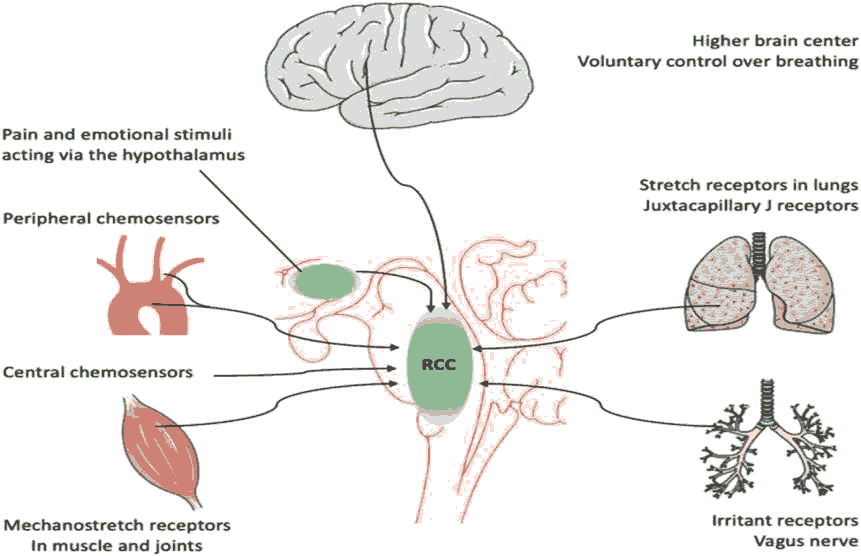
Control of ventilation and responses to environmental and physiological drivers of dyspnea are complex [1]. Neural signaling to the brain regarding breathing includes: a) chemoreception by the peripheral and central chemoreceptors of arterial oxygen pressure (PaO2) , pH and arterial partial pressure of carbon dioxide (PaCO2), and b) afferent signaling from the lungs, respiratory muscles and chest wall regarding muscle effort, depth of breathing, lung stretch, and inflammation to the brainstem respiratory control center and its ‘corollary projection’ to higher cortical centers such as the amygdala and anterior insular cortex, where the conscious sensation of breathing resides [9] (Figure 1).
If Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has a direct effect on peripheral oxygen-sensing and response given the possibility of the presence of Angiotensin Converting Enzyme 2 (ACE-2) receptors in the carotid body [10] and elsewhere in the central nervous system [11]; a direct virally-mediated effect at the level of the carotid bodies could potentially limit the ventilatory response to hypoxia, and within the midbrain and higher cortical sensory areas could decrease or abolish the sense of dyspnea. Recent autopsy findings in patients with COVID-19 has shown evidence for both SARS-CoV-2 RNA and protein in many areas of the brainstem and cortex often but not always associated with neuropathological changes [12]. Relatively asymptomatic COVID-19 patients with hypoxemia can have rapid respiratory decompensation and greater mortality [13].
In certain patients, the early stages of COVID-19 were associated with severe silent hypoxemia, despite mild parenchymal lung involvement [14,15]. The pronounced arterial hypoxemia yet without proportional signs of respiratory distress was referred to as silent or ‘happy’ hypoxemia. Silent hypoxemia can occur with PaO2 ranging between 36 mmHg and 45 mmHg in the absence of increased alveolar ventilation. This phenomenon is becoming important in some patients with COVID-19, where the severity of hypoxemia can be an important predictor for patient admission to the Intensive Care Unit (ICU).
The correct recognition of hypoxemia has such an impact on prognosis and timely treatment decisions; therefore, this review focuses on understanding the causality of this phenomenon in COVID-19 patients specifically. Correcting hypoxemia in patients with refractory hypoxemic respiratory failure calls for invasive ventilation support that might be superior to non-invasive ventilation in boosting transpulmonary pressure, opening collapsed alveoli, and improving oxygenation. In order to manage the profound hypoxemic respiratory failure and the clinically apparent patient’s wellbeing, respiratory rate, hyperventilation signs and oxygen saturation. Additionally, invasive measurements of PaO2/ PaCO2 need to be closely monitored, at regular time intervals. The arterial hypoxemia can be induced by intrapulmonary shunting, dysregulated hypoxic pulmonary vasoconstriction, impaired lung diffusion, and formation of intravascular microthrombi.
Attempting to explain this phenomenon, Gattinoni et al. [16] identified two COVID-19 phenotypes: Type L, with limited ground-glass opacification on computed tomography (CT) of the chest which seemed responding to lower Positive End-Expiratory Pressure (PEEP), and type H, with more widespread opacification on CT, which was possibly benefiting from higher (PEEP) [17]. However, Boss and colleagues found no relationship between lung compliance and qualitative CT measurements, suggesting that these phenotypes may not be mutually exclusive [18].
Literature Review
Pulmonary Vasculopathy in COVID-19 Patients
In some cohorts of patients with COVID-19, the feature of vascular thickening was observed on chest CT [19]. Moreover, the presence of small, firm thrombi in the peripheral parenchyma of the lungs, in the absence of thrombi in the main pulmonary arteries was described in post-mortem series of patients with COVID-19 [20]. Other post- mortem studies have described perivascular lymphocytic inflammation in patients with SARS-CoV-2 [21]. Notably, some patients infected with SARS-CoV-2 were found to have cutaneous vasculitis [22]. Such endotheliitis and vasculitis may cause severe damage to the vascular endothelia and, therefore, increase the risk of thrombosis [19]. The presence of microthrombi, endotheliitis and vasculitis in the peripheral pulmonary arteries may cause pulmonary hypertension.
Silent hypoxemia in COVID-19
Hypoxemia plays a limited role in the sensation of breathlessness and usually experienced by patients with cardiopulmonary disease. In healthy subjects, who are staying at high altitude, or entering experimental hypoxic chambers might have some respiratory drive shifts towards mild hypoxemia, i.e. PaO2 60 mmHg–65 mmHg. In order to deal with hypoxemia, a rise in minute ventilation, in tidal volume and respiratory rate is usually recommended.
Nevertheless, the silent hypoxemia phenomenon of COVID-19 patients raised the possibility of other type of hypoxemia. This is supported by the findings of Guan et al. [21] who reported abnormal CT scans in only 86% of their cohort of 1,099 hospitalized COVID-19 patients, despite having a low PaO2 to fraction of inspired oxygen ratio (FiO2) (PaO2/ FiO2 ratio) [24]. Our suggested mechanism for silent hypoxemia in patients with COVID-19 is the diversion of part of the pulmonary circulation towards non-aerated areas of the lungs by peripheral pulmonary thrombi. As a result, ventilation-perfusion mismatch occurs, causing vascular shunt and hypoxemia [22]. In a cohort with COVID-19, a shunt fraction of 50% was reported with an average of non-aerated lung fraction of 17% as evidenced by chest CT. The shunt fraction reported in COVID-19 disease was found to be higher than the shunt fraction in found in ordinary ARDS [15,24].
Dhont et al. [23] reported that COVID-19 patients presented a disconnect between profound hypoxemia and signs of respiratory distress (i.e. silent hypoxemia). Some patients showed rapid deterioration that led to higher referral to the intensive care unit. Dhont et al. called for thorough understanding of the pathophysiological determinants of respiratory drive and hypoxemia that would help to clinically manage the patients more effectively. Dhont et al. explained the preserved oxygen saturation with low PaO2 by the shift of the oxyhemoglobin dissociation curve induced by hypoxemia-driven hyperventilation, besides the possible role of viral interactions with hemoglobin.
Summary of the Studies Reporting Pulmonary Vasculopathy in Patients with COVID-19
In a series of CT chest scans performed in 45 patients with COVID-19, Spagnolo et al. [25] reported a substantial increase in both the pulmonary artery diameter and the ratio of pulmonary artery diameter to ascending aorta diameter after acquiring COVID-19 infection. Surprisingly, the reported increase in pulmonary artery diameter was not correlated with the extent of pneumonia severity, however, it was correlated with higher mortality.
Similarly, Lang et al. [26] reported abnormally dilated vessels inside and outside lung opacities in 41/45 of hospitalized patients with COVID-19. Pulmonary emboli, however, were only present in 7/45 patients. Additionally, perfusion abnormalities could be detected by the use of dual energy chest CT. Comparably, Patel et al. [27] noticed major vascular perfusion irregularities and increased physiologic dead space associated with hypercoagulability in severe COVID-19 pneumonia. Dilated peripheral vessels were observed in 21/33 patients, however, acute pulmonary embolism was found in only 10/21 patients. Similarly, they reported perfusion defects in dual energy chest CT.
On the other hand, Grillet et al. [28] diagnosed acute pulmonary embolism in 23/100 patients with severe COVID- 19 and Pagnesi et al. [29] could diagnose pulmonary hypertension by transthoracic echocardiography in 24/200 hospitalised patients with severe COVID-19 which was associated with higher mortality.
‘Silient hypoxemia’ as Untapped Solution
Buheji and Ahmed [30] referred to the value of various untapped solutions that comes from holistic multidisciplinary input perspectives when dealing with COVID-19 pandemic and its related life-threatening issues [31]. The pressure developed after overwhelming the healthcare institutions, specifically ICUs’ can be avoided if the COVID-19 hidden characteristics or its ways of influencing patients were known. In this review, knowing the proper mechanism(s) that would clarify the mechanism of silent hypoxemia, would help to improve the overall effectiveness of the treatment and reduce the patients’ mortality before and after ICU admission [32,33].
Besides the published research work that shows pathophysiological abnormalities in COVID-19 patients that explains the disconnect between the severity of hypoxemia and the relatively mild respiratory discomfort; this review clarifies the vascular mechanisms occurring in those patients. The vascular mechanisms can explain the phenomenon of silent hypoxemia in COVID-19 patients due to the occurrence of pulmonary vasculopathy which increases the shunt fraction and causes ventilation/perfusion mismatch.
The implication of this research is that it opens a path for future research in patients with severe COVID-19 who had been reported to have significantly elevated levels of tissue plasminogen activator and plasminogen activator inhibitor-1. Consequently, they are prone to have vascular thrombi in the pulmonary vessels which can be diagnosed by dual energy CT scanning of the chest [33].
Hence, this narrative review suggests that fibrinolytics should continue to be investigated as therapeutic avenue in patients with COVID-19 especially if they presented with silent hypoxemia without evidence of pulmonary parenchymal infiltrates or without evidence pulmonary embolism in the main pulmonary arteries. Silent hypoxemia can be very dangerous as it is not associated with dyspnea which alarms both the patients and the care givers. Great attention should be given to patients with silent hypoxemia to avoid the silent death. Routine monitoring of the oxygen saturation may be essential in asymptomatic COVID-19 patients to detect silent hypoxemia. Silent hypoxemia should be thoroughly investigated and the use of thrombolytic therapy should be initiated once the diagnosis of pulmonary microthrombi was confirmed.
Conclusion
Pulse oximetry should be used at the early stages of COVID-19 disease to detect silent hypoxemia. Dual energy CT scanning of the chest can be used to diagnose vascular thrombi in the pulmonary vessels if no clear explanation of the hypoxemia was found. Future research should test the value of using systemic thrombolytics to treat vasculopathy related severe hypoxemia in COVID-19 disease.
References
- Parshall, M., et al. “An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea.” Am J Respir Crit Care Med 185.4 (2012): 435-452.
- Tobin, M. J., et al. “Why COVID-19 silent hypoxemia is baffling to physicians.” Am J Respir Crit Care Med 202.3 (2020): 356–360.
- Ottestad, William., and Sovik, Signe. “COVID-19 patients with respiratory failure: What can we learn from aviation medicine?” Br J Anaesth 125.3 (2020): e280–e281.
- Nishino, T. “Dyspnoea: Underlying mechanisms and treatment.” Br J Anaesth 106.4 (2011), 463–474.
- Chen, Zibin., et al. “Respiratory-associated thalamic activity is related to level of respiratory drive.” Respir Physiol 90.1 (1992): 99–113.
- Tung, Hui. Ying., et al. “Advances and evolving concepts in allergic asthma.” Semin Respir Crit Care Med 39.1 (2018): 64–81.
- Galeas, Pena. M., McLaughlin, N., and Pociask, Derek. “The role of the innate immune system on pulmonary infections.” Biol Chem 400.4 (2019): 443–456.
- Malhotra, Atul. “Low-tidal-volume ventilation in the acute respiratory distress syndrome.” N Engl J Med 357. 11 (2007), 1113–1120.
- Stoeckel, M. Corrnelia., et al. “Brain mechanisms of shortterm habituation and sensitization toward dyspnea.” Front Physiol. 6 (2015): 748.
- Fung, Man. Lung. “The role of local renin-angiotensin system in arterial chemoreceptors in sleepbreathing disorders.” Front Physiol 5 (2014): 336
- Kabbani, N., Olds, James L. “Does COVID19 infect the brain? If so, smokers might be at a higher risk.” Mol Pharmacol 97.5 (2020): 351-353.
- Matschke, Jakob., et al. “Neuropathology of patients with COVID-19 in Germany: A post-mortem case series.” Lancet Neurol 19.11 (2020): 919-929.
- Xie, Jang., et al. “Association between hypoxemia and mortality in patients with COVID-19.” Mayo Clin Proc 95.6 (2020): 1138-1147.
- Bos, Lieuwe. D.,et al. “Sub-phenotyping Acute Respiratory Distress Syndrome in patients with COVID-19 Patients: Consequences for ventilator management.” Ann Am Thorac Soc 17.9 (2020): 1161-1163.
- Gattinoni, Luciano., et al. “Covid-19 does not lead to a typical acute respiratory distress syndrome.” Am J Respir Crit Care Med 201.10 (2020): 1299–1300.
- Gattinoni, Luciano., et al. “COVID-19 pneumonia: Different respiratory treatments for different phenotypes?.” Intensive Care Med 46.6 (2020): 1099-1102.
- Bai, H. X. et al. (2020) Performance of radiologists in differentiating COVID-19 from non- Covid-19 viral pneumonia at chest CT. Radiology 296.2 (2020):e46-e54.
- Fox, Sharon. E., et al. “Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans.” Lancet Respir Med 8.7 (2020): 30243-30245.
- Ackermann, Maximillian., et al. “Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19.” N Engl J Med 383 (2020): 120-128.
- Castelnovo, L., “Symmetric cutaneous vasculitis in COVID-19 pneumonia.” J Eur Acad Dermatology Venereol 34.8: e362-e363.
- Guan, Wei., et al. “Clinical characteristics of coronavirus disease 2019 in China.” N Engl J Med 382 (2020), 1708–1720.
- D’Alonzo, Gilbert. E., et al. “Respiratory failure, mechanisms of abnormal gas exchange, and oxygen delivery.” Med Clin North Am 67.3 (1983): 557–571.
- Dhont, Sebastiaan., et al. “The pathophysiology of ‘happy’ hypoxemia in COVID-19.” Respir Res 21.1 (2020): 198.
- Gattinoni, Luciano., et al. “Lung recruitment in patients with the acute respiratory distress syndrome.” N Engl J Med 354.17 (2006): 1775–1786.
- Spagnolo, Pietro., et al. “CT-derived pulmonary vascular metrics and clinical outcome in COVID-19 patients.” Quant Imaging Med Surg 10.6: 1325–1333.
- Lang, Min., et al. “Pulmonary vascular manifestations of COVID-19 pneumonia.” Radiol Cardiothorac Imaging 2.3: e200277.
- Patel, B. V et al. (2020) “Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations.” Am J Respir Crit Care Med 202 (2020): 690–699.
- Grillet, Franck., et al. “Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography.” Radiology 296.3 (2020).
- Pagnesi, Matteo., et al. “Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19.” Heart 106.17 (2020): 1324–1331.
- Woyke, Simon., et al. “Modulation of Hb-O2 affinity to improve hypoxemia in COVID-19 patients.” Clin Nutr 40.1 (2021): 38-39.
- Buheji, Mohamed., Cunha, Katiane., and Rocha, Rodrigo.“Ventilators in COVID-19, between scarcity and abundance mindset.” IJARET 11.10 (2020): 751-767.
- Buheji, Mohamed., et al. “Re-inventing the intensive care units capacity in response to covid-19 pandemic second wave.” Int J Manag 11.10: 914-923.
- Henry, Brandon. M., et al. “Circulating levels of tissue plasminogen activator and plasminogen activator inhibitor-1 are independent predictors of coronavirus disease 2019 severity: A prospective, observational study.” Semin Thromb Hemost (2021).