Research - International Journal of Medical Research & Health Sciences ( 2022) Volume 11, Issue 3
Status of H2AX and TP53 (Non- phosphorylation) for DNA Damage in Cancer Patients on Radiotherapy-A Case-Control Study
Alkhansa S. Mahmoud1*, Omer M. Abdalla1,4, Amna A. Ali1, Nuha M. Hassan1, Amna A. Yousif1, Fawzia E. Elbashir2, Ahmed Omer3, Raheel Mussa5 and Ammar ME. Hassan12Medical Physics Department, National Cancer Institute, University of Gezira, Wad Medani, Sudan
3Radiation and Isotope Centre, Khartoum, Sudan
4Department of Biochemistry, Nile College, Khartoum, Sudan
5University of Gezira, Wad Medani, Sudan
Alkhansa S. Mahmoud, Radiobiology Department, Sudan Atomic Energy Commission, Khartoum, Sudan, Email: Alkhansa.salih@gmail.com
Received: 02-Mar-2022, Manuscript No. ijmrhs-22-57321 (M); Editor assigned: 04-Mar-2022, Pre QC No. ijmrhs-22-57321 (P); Reviewed: 17-Mar-2022, QC No. ijmrhs-22-57321 (Q); Revised: 20-Mar-2022, Manuscript No. ijmrhs-22-57321 (R); Published: 31-Mar-2022
Abstract
Introduction: Radiotherapy is widely used in the treatment of cancer patients, and some genes affect the response to radiotherapy. In addition, phosphorylation is a hallmark of DNA damage response. In this study, we aim to investigate the status of H2AX and the non-phosphorylation forms of tumor suppressor p53 (TP53) before and after radiotherapy to reveal their roles in DNA damage response and radio-sensitivity. Materials and Methods: ELISA technique was used to determine the H2AX concentration and TP53 expression status in plasma of 29 cancer patients and 29 healthy individuals of the same age and sex as a control group. Patients were treated with radiotherapy, while the control group was not irradiated. H2AX and TP53 were measured before the start of radiotherapy on the first day of treatment and then 30 minutes after radiotherapy on the last day. Results: Both H2AX and TP53 had higher levels in cancer patients before radiotherapy than in healthy subjects. In 21 patients, H2AX levels were significantly decreased after radiotherapy compared with levels before radiotherapy (p=0.002), and TP53 levels were significantly increased after radiotherapy compared with levels before radiotherapy (p=0.0008). In eight patients (n=8), H2AX levels were increased after radiotherapy and TP53 expression was decreased after radiotherapy. Conclusion: The results show that both H2AX and TP53 can be induced by DNA damage. The decrease of H2AX level after radiotherapy refers to the conversion of this histone protein to the phosphorylated form (gamma-H2AX) after radiotherapy, so H2AX can be used as an indicator of DNA damage response and radiation sensitivity by the phosphorylation form (gamma-H2AX) in early exposure. While TP53 is activated by radiotherapy because its expression is increased after radiotherapy in cancer patients, indicating that TP53 is not yet phosphorylated, TP53 can also be used as a marker to indicate DNA damage response in early radiation exposure.
Keywords
H2AX, Radiotherapy, TP53, DNA damage
References
- Chierchini, Sara, et al. "Physician and patient barriers to radiotherapy service access: Treatment referral implications." Cancer Management and Research, Vol. 11, 2019, pp. 8829-33.
Google Scholar Crossref - Tang, Le, et al. "Role of metabolism in cancer cell radioresistance and radiosensitization methods." Journal of Experimental & Clinical Cancer Research, Vol. 37, No. 1, 2018, pp. 1-15.
Google Scholar Crossref - Huang, Rui-Xue, and Ping-Kun Zhou. "DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer." Signal Transduction and Targeted Therapy, Vol. 5, No. 1, 2020, pp. 1-27.
Google Scholar Crossref - Bartova, Eva, et al. "Histone modifications and nuclear architecture: A review." Journal of Histochemistry & Cytochemistry, Vol. 56, No. 8, 2008, pp. 711-21.
Google Scholar Crossref - Redon, Christophe E., et al. "γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin." Advances in Space Research, Vol. 43, No. 8, 2009, pp. 1171-78.
Google Scholar Crossref - Zlobinskaya, O., et al. "Induction and repair of DNA double-strand breaks assessed by gamma-H2AX foci after irradiation with pulsed or continuous proton beams." Radiation and Environmental Biophysics, Vol. 51, No. 1, 2012, pp. 23-32.
Google Scholar Crossref - Srivastava, Niloo, et al. "Copy number alterations of the H2AFX gene in sporadic breast cancer patients." Cancer Genetics and Cytogenetics, Vol. 180, No. 2, 2008, pp. 121-28.
Google Scholar Crossref - Bassing, Craig H., et al. "Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX." Proceedings of the National Academy of Sciences, Vol. 99, No. 12, 2002, pp. 8173-78.
Google Scholar Crossref - Parikh, Rahul A., et al. "Loss of distal 11q is associated with DNA repair deficiency and reduced sensitivity to ionizing radiation in head and neck squamous cell carcinoma." Genes, Chromosomes and Cancer, Vol. 46, No. 8, 2007, pp. 761-75.
Google Scholar Crossref - Levine, Arnold J. "p53: 800 million years of evolution and 40 years of discovery." Nature Reviews Cancer, Vol. 20, No. 8, 2020, pp. 471-80.
Google Scholar Crossref - Lindsay, K. J., et al. "The genetic basis of tissue responses to ionizing radiation." The British Journal of Radiology, Vol. 80, No. special_issue_1, 2007, pp. S2-S6.
Google Scholar Crossref - Takagi, Masatoshi, et al. "Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin." Cell, Vol. 123, No. 1, 2005, pp. 49-63.
Google Scholar Crossref - Lee, Chang-Lung, Jordan M. Blum, and David G. Kirsch. "Role of p53 in regulating tissue response to radiation by mechanisms independent of apoptosis." Translational Cancer Research, Vol. 2, No. 5, 2013, pp. 412-21.
Google Scholar Crossref - Kirkpatrick, John P., et al. "The radiosurgery fractionation quandary: Single fraction or hypofractionation?" Neuro-oncology, Vol. 19, No. suppl_2, 2017, pp. ii38-ii49.
Google Scholar Crossref - Baskar, Rajamanickam, et al. "Cancer and radiation therapy: Current advances and future directions." International Journal of Medical Sciences, Vol. 9, No. 3, 2012, pp. 193-99.
Google Scholar Crossref - Saeed, Mohamed EM, et al. "A five-year survey of cancer prevalence in Sudan." Anticancer Research, Vol. 36, No. 1, 2016, pp. 279-86.
Google Scholar Crossref - Redon, Christophe E., et al. "γ-H2AX detection in peripheral blood lymphocytes, splenocytes, bone marrow, xenografts, and skin." DNA Damage Detection In Situ, Ex Vivo, and In Vivo, 2011, pp. 249-70.
Google Scholar Crossref - Sproull, Mary, and Kevin Camphausen. "State-of-the-art advances in radiation biodosimetry for mass casualty events involving radiation exposure." Radiation Research, Vol. 186, No. 5, 2016, pp. 423-35.
Google Scholar Crossref - Withers, H. Rodney. "The four R's of radiotherapy." Advances in Radiation Biology, Vol. 5, 1975, pp. 241-71.
Google Scholar Crossref - Fowler, John F. "The linear-quadratic formula and progress in fractionated radiotherapy." The British Journal of Radiology, Vol. 62, No. 740, 1989, pp. 679-94.
Google Scholar Crossref - Ivashkevich, Alesia, et al. "Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research." Cancer Letters, Vol. 327, No. 1-2, 2012, pp. 123-33.
Google Scholar Crossref - Ben Kacem, Mariam, et al. "Variation of 4 MV X-ray dose rate in fractionated irradiation strongly impacts biological endothelial cell response in vitro." International Journal of Radiation Biology, Vol. 98, No. 1, 2022, pp. 50-59.
Google Scholar Crossref - Wang, Lihua H., et al. "Monitoring drug-induced γH2AX as a pharmacodynamic biomarker in individual circulating tumor cells." Clinical Cancer Research, Vol. 16, No. 3, 2010, pp. 1073-84.
Google Scholar Crossref - Vousden, Karen H., and Carol Prives. "Blinded by the light: The growing complexity of p53." Cell, Vol. 137, No. 3, 2009, pp. 413-31.
Google Scholar Crossref - Kong, Xiaohan, et al. "Relationship between p53 status and the bioeffect of ionizing radiation." Oncology Letters, Vol. 22, No. 3, 2021, pp. 1-8.
Google Scholar Crossref
Introduction
A radiotherapy is a primary option for the treatment of cancer patients. It is used either alone or in combination with surgery or chemotherapy to inhibit tumour development [1]. During radiotherapy, some tumour cells may develop radioresistance, which affects the response to radiotherapy. However, radioresistance is related to epigenetics, which affects cell regulation [2]. DNA double-strand breaks induced by ionizing radiation can trigger a series of cellular DNA damage responses such as cell cycle arrest and DNA repair [3]. H2AX is one of several genes encoding the histone H2A family. In humans and other eukaryotes, DNA is wrapped around histone groups in chromatin consisting of core histones H2A, H2B, H3, and H4 [4]. H2AX is phosphorylated at serine 139 and then forms gamma-H2AX, in response to DNA Double-Strand Breaks (DSBs) after ionizing radiation exposure [5]. H2AX increases with time after irradiation and reaches a maximum (20-30) min after irradiation [6]. The human H2AX gene (H2AFX) is mapped to chromosome 11 at position 11q23, in a region that frequently has mutations in a large number of human cancers [7]. In squamous cell carcinomas of the head and neck, amplification occurs in chromosomal region 11q13 followed by loss of distal 11q, the region containing H2AFX [8]. The increased chromosomal instability in these cells is associated with the loss of 11q and H2AX, which contributes to progression, resistance, and tumour development in this cancer subtype [9]. In contrast, the tumour suppressor P53 is key to various stress signals in cellular responses such as DNA damage, reactive oxygen species, apoptosis, and senescence [7]. Tumour suppressor P53 is an important regulator of cellular response to radiation [10]. TP53 is a multifunctional transcription factor; upon radiation exposure, the level of P53 protein increases in cells due to activation of the DNA damage response [11]. Activation of p53-mediated signalling pathways facilitates DNA repair and initiates the intrinsic pathway of apoptosis. Therefore, P53 plays an important role in controlling cell activity after irradiation [12]. Therefore, TP53 expression in cancer cells after radiotherapy could be associated with the efficacy of radiotherapy [13]. Therefore, understanding the TP53 status in the response of a cancer cell to irradiation could help to solve the problem of radioresistance and improve the outcomes of radiotherapy.
Total dose fractionation is a powerful technique used in modern radiotherapy [14]. It involves dividing the total dose to the patient into small doses that are administered separately and then recover over hours (approx. 12-24 hours), which helps both cancer cells and normal cells respond to radiation [15]. However, the recovery of the previous dose infractions requires hours, so the levels of H2AX and TP53 after radiotherapy usually indicate DNA damage 30 minutes after the last dose. In the present study, H2AX and TP53 status were determined 30 minutes after radiotherapy to understand their roles in DNA damage in radiotherapy, which could serve as biomarkers for radiation sensitivity and therapeutic response monitoring to improve the outcomes of radiotherapy in cancer patients.
Materials and Methods
Study Population
A total of 29 cancer patients undergoing radiotherapy participated in this study after obtaining ethical approval from the competent authority. Blood samples were collected before and 30 minutes after the last session of radiotherapy total dose fractions. Twenty-nine healthy individuals who were not exposed to radiation voluntarily participated in this study as a control group and matched the patients in age and sex. The participating patients were treated at the National Cancer Institute Clinic, the second centre for cancer treatment in Sudan.
Included Criteria of Cancer Patients
Patients undergo radiotherapy as a second line of treatment either after chemotherapy or surgery. Patients were selected according to two cancer types: breast, head, and neck cancer, as breast cancer is classified as one of the most common cancers in Sudanese women with increasing incidence, while head and neck cancer are the most commonly diagnosed cancers in men, women, and children <15 years [16].
External Beam Radiotherapy
Cancer patients treated with Cobalt 60. A conventional simulator planning system was used to adjust the radiation dose as needed to ensure that the beam was accurately focused on the tumour with minimal exposure to normal tissue. All patients were treated at early detection with an α/β ratio of 10.
Collection and Preparation of Blood Samples
Blood samples (3 ml) were collected from cancer patients and healthy donors matched for age and sex. Blood samples were collected in EDTA vacutainer tubes and then separated by centrifugation at 2500 rpm for 10 min at room temperature to collect plasma and then stored at -20℃ until analysis.
Reagents
Human H2AX Enzyme-Linked Immunosorbent Assay (ELISA) kits from Lifespan Bioscience, Inc. Company were used to measure H2AX concentrations in plasma of patients and control, and the kits were developed for research purposes only. The assay is based on the sandwich ELISA principle. H2AX was detected by using specific monoclonal and polyclonal antibodies against H2AX in human plasma samples. The assay was performed in two runs, then the mean value was determined calorimetrically at 450 nm and the match was established (Pg/ml).
The TP53 antibody used against non-phosphorylation of TP53 was from CUSABIO (CSB-PA003677). The methodology was based on the protocol corresponding to the principle of the ELISA technique. Subsequently, the optical density was determined at 450 nm.
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism 8 software (Graph Pad Software Inc., San Diego, California, USA). The student’s t-test (paired and unpaired) was used to determine the significant differences in H2AX values between groups. Significance was considered at the p<0.05 level.
Results
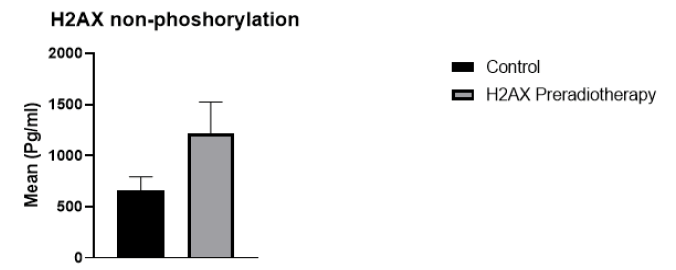
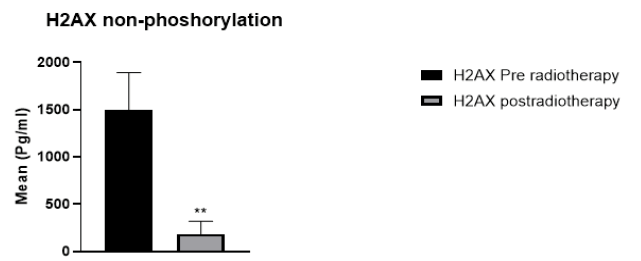
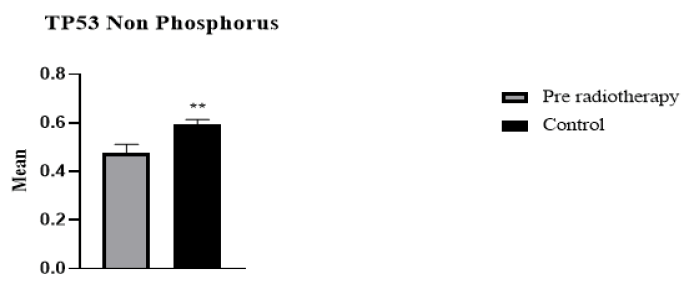
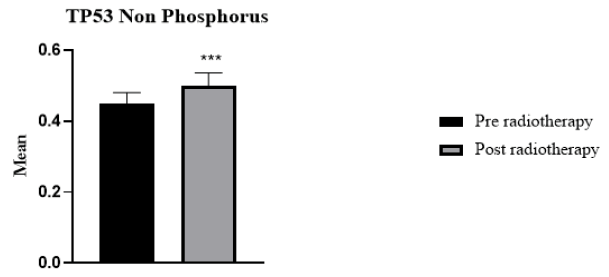
29 cancer patients participated in this study, 20 breast cancer patients, and 9 patients with head and neck cancer, including oesophagus, lip, mandible, nasopharynx, and thyroid. The patients were 6 male and 22 female. The age range of breast cancer patients was 30 to 65 years. For head and neck cancer patients, the age range was 12-77 years. The absorbed dose for breast cancer was 40.5 Gy (2.7 Gy/day). For head and neck cancer, the dose ranged from 20 Gy66 Gy (2 Gy/day). Regarding H2AX levels in cancer patients compared to the control group, H2AH levels were higher in cancer patients than in the healthy group (Figure 1). Whereas H2AX significantly decreased in cancer patients after radiotherapy compared with the values before radiotherapy (p=0.002) (Figure 2). Of the total patients, 21 patients showed a decrease in H2AX levels after radiotherapy at 30 minutes of total dose fractions. In 8 patients, H2AX levels were increased after radiotherapy and TP53 levels decreased after radiotherapy; four of the eight patients were head and neck tumours. TP53 non-phosphorus level significantly increased in cancer patients compared with the control group (p=0.006) (Figure 3). TP53 also increased significantly in 21 patients after radiotherapy compared with levels before radiotherapy (p=0.0008) (Figure 4).
Discussion
Biomarkers to monitor the efficacy of radiotherapy in cancer patients are essential for radiation oncology. H2AX has become an important tool for monitoring DSBs in translational cancer research. Previous studies have used various techniques to assess the response to DSBs using H2AX [17]. Researchers in radiation biology are focusing their attention on radiation biodosimetry, emphasizing the potential of the assay to determine the radiosensitivity of patients [18]. Moreover, in the present study, the total amount of H2AX and TP53 status were investigated based on the ELISA principle. For total H2AX, H2AX levels decreased after radiotherapy compared with levels before radiotherapy, suggesting that the decay of H2AX after radiotherapy is due to the DNA damage response caused by radiotherapy, but also that H2AX levels decrease or fall because H2AX is phosphorylated in the form of γ-H2AX after irradiation. Several studies on radiation-induced γ-H2AX reported that H2AX foci were observed on fibroblasts and lymphocytes after acute external irradiation [19]. In another in vitro study, the decay of γ-H2AX foci was found to correlate with the repair of lethal damage, which is part of the radiation damage that can be affected after irradiation in prostate cancer cell lines [20]. Several studies reported that H2AX is increased in cancer patients compared with normal patients, confirming our findings [21]. As expected, H2AX levels could show the effects of DNA damage after radiotherapy, indicating radio-sensitivity after total dose fractionation. In this study, some patients (n=8) showed increased H2AX levels after radiotherapy, explaining that H2AX, which is not phosphorylated, could be activated by radiation. Moreover, activation of the non-phosphorylated form in these patients seems to be responsible for a low DNA damage response after 30 minutes, because phosphorylation is a hallmark of the DNA damage response. However, the Biologically Effective Dose (BED) in particular is useful to quantify treatment expectations, such that patients treated with a high α/β ratio (e.g., 10 Gy) may indicate radiation resistance and increased morbidity [22].
As for TP53 expression status, the present study showed that radiotherapy activated TP53 (non-phosphorus) and its expression increased after radiation exposure in cancer patients. Several studies confirmed that TP53 is induced by radiation [23]. TP53 is an important tumour suppressor gene that is expressed at low levels in cells [24]. However, when cells are stressed by toxic agents such as radiation, the expression of P53 increases. This mechanism plays an important role in maintaining the genomic stability of the cell and inhibiting carcinogenesis [25]. These findings explain the role of H2AX and TP53 in DNA damage response after early radiotherapy such as 30 minutes. These results show that H2AX and TP53 are in a non-phosphorylated form that is activated by radiation. This explains that TP53 can be used for DNA damage response after radiotherapy. However, H2AX can be used to detect DNA damage response as early as 30 min after radiotherapy, and TP53 activation is strongly induced by DNA damage and drives the response to radiation. The present results demonstrate the potential effects of H2AX and TP53 on DNA damage response after radiotherapy. Furthermore, understanding the responses of cancer cells after radiotherapy will potentially lead to improved therapeutic efficacy. The importance of radio-sensitivity in radiation oncology is critical to monitor drug response and increase the accuracy of the absorbed dose delivered to the patient. In addition, the development of biomarkers for radio sensitivity is needed.
Conclusion
The decreased H2AX levels after radiotherapy refer to the conversion of H2AX to the phosphorated form (gamma H2AX) after radiotherapy. Thus, H2AX can be used to detect DNA damage after radiotherapy, and the phosphorylated form gamma H2AX can be used to monitor radiation sensitivity in cancer patients. On the other hand, the increased expression of TP53 (without phosphorylation) after radiotherapy suggests that this protein is activated by radiation and induced by DNA damage, which explains its role in DNA damage response, so TP53 can be used as a marker of DNA damage response in early radiotherapy.
Declarations
Ethical Approval and Consent to Participate
The proposal was approved by the Ethics Committee of the Research Centre of the Federal Ministry of Health. All patients were informed of their concerns by a written individualized form, except for one child <15 years of age who was consented to by his or her parents.
Consent for Publication
Consent was obtained for publication.
Competing Interests
The authors declared no conflict of interest.
Funding
The work is supported by IAEA, Contract Research Project Grant/IAEA CRP agreement. This work is part of IAEA Coordinated Research Project E35010 entitled “Applications of Biological Dosimetry Methods in Radiation Oncology, Nuclear Medicine, and Diagnostic and Interventional Radiology (MEDBIODOSE).”
Author Contributions
Alkhansa designed the study, performed the statistical analysis, wrote the article, and received funding. Ammar ME. Hassan contributed to sample preparation, interoperation results, and review of the paper. Amna Yousif and Raheel Mussa performed the patient consent forms and sample collection. Omer M. Abdalla, Amna Ali, and Nuha M. Hassan contributed to the analysis of the samples. Fawzia Elbashir and Ahmed Omer were involved in the selection criteria for the patients in the clinic and radiotherapy department.