Research - International Journal of Medical Research & Health Sciences ( 2022) Volume 11, Issue 1
The Hazard of Developing Adverse Reactions in Rheumatoid Arthritis Patients receiving Intravenous Biologics: A Survival Analysis
Haya M. Almalag1*, Shiekha S. AlAujan1, Hadeel Alkofide1, Hawazin S. Alhazzani1, Lamia A. Alzamel1, Reem S. Tashkandi1, Hussain F. Alarfaj2, Abdurhman S. Alarfaj2, Hanan H. Abouzaid1, Ali M. Alhadri2,3 and Mohammed A. Omair22Rheumatology Unit, Department of Medicine, King Saud University Medical City, Riyadh, Saudi Arabia
3Rheumatology Unit, Armed Forces Hospital, Southern Region, Saudi Arabia
Haya M. Almalag, Clinical Pharmacy Department, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia, Email: halmalaq@ksu.edu.sa
Received: 03-Jan-2022, Manuscript No. ijmrhs-22-51178 (M); Accepted Date: Jan 21, 2022 ; Editor assigned: 05-Jan-2022, Pre QC No. ijmrhs-22-51178 (P); Reviewed: 11-Jan-2022, QC No. ijmrhs-22-51178 (Q); Revised: 18-Jan-2022, Manuscript No. ijmrhs-22-51178 (R); Published: 27-Jan-2022
Abstract
Objectives: Limited previous studies have investigated the hazard and time to develop Adverse Drug Reactions (ADRs) of Intravenous (IV) biological Disease-Modifying Antirheumatic Drugs (bDMARDs) in patients with Rheumatoid Arthritis (RA). This study aimed to investigate the hazard of using different IV originator bDMARDs in comparison to each other and to assess the survival of this hazard in RA patients with or without conventional synthetic Disease- Modifying Antirheumatic Drugs (csDMARDs). Methods: This is a single-centre retrospective cohort study at in Saudi Arabia, of adult RA patients receiving IV originator bDMARDs, including Tocilizumab (TCZ), Rituximab (RTX), Abatacept (ABA), and Infliximab (IFX), with or without conventional synthetic Disease-Modifying Antirheumatic Drugs (csDMARDs). Data extraction was between January 2019 to March 2020. The type and time to develop ADRs for each bDMARDs between 2015-2020 were noted. Results: A total of 129 were eligible for inclusion with 113 (87.6%) were females with a mean age of 54 (SD ± 13) years. Patients received TCZ (38.8%) RTX (38.8%), ABA (14.0%) and IFX (8.5%). A significant difference was observed in the ADR-free survival period among the included bDMARDs (p=0.01) with ABA as a reference group. RTX was associated with lower ADRs in 1500 days of follow-up compared with ABA (Hazard Ratios (HRs)=0.39, p=0.003), followed by TCZ (HR=0.45, p=0.012). This remained significant even after the adjustment for confounders. IFX had lower ADRs compared with ABA, but this result was statistically insignificant. Conclusion: There is significantly less hazard of developing ADRs while using IV bDMARDs, namely, TCZ and RTA, compared with ABA in RA patients.
Keywords
Intravenous biologic DMARDs, Adverse drug reactions, Rheumatoid arthritis, Survival, Hazard
Introduction
Rheumatoid Arthritis (RA) is a progressive inflammatory disorder affecting the joints and can lead to deformities. Treatment with Disease-Modifying Antirheumatic Drugs (DMARDs) is essential in delaying the progression of bone erosions and improving patients’ quality of life [1]. According to the 2015 guidelines of the American College of Rheumatology (ACR) for the treatment of RA, biologic DMARDs (bDMARDs) are now considered to be the standard of care for patients with an inadequate response to conventional synthetic Disease-Modifying Drugs (csDMARDs). Advances in the management of RA led to the frequent use of these agents and a greater risk of facing safety issues [2-4]. The World Health Organization (WHO) defined an Adverse Drug Reaction (ADR) as ‘any response to a drug which is noxious and unintended, and which occurs at doses used in man for prophylaxis, diagnosis or treatment’ [5]. As chronic management in RA is essential, the risk for ADRs is always present [6]. However, the existing literature discussing these ADRs is limited. bDMARDs differ from each other in terms of the route and frequency of administration. Intravenous (IV) administration of bDMARDs, such as Infliximab (IFX), Rituximab (RTX), Abatacept (ABA), and Tocilizumab (TCZ), poses a more significant concern for patients due to perceived harm and clinicians due to the risk of infusion-related reactions [7].
A previous meta-analysis and a recent systematic review discussed the safety of various bDMARDs with different administration routes in head-to-head comparisons. However, the heterogeneity between these studies due to the variability in study designs and definition of ADRs compromised their credibility [8,9]. The authors observed that randomized controlled trials are generally limited by the small sample size and short follow-up period. Whereas, observational studies can assess patients for a longer period, thus capturing less common ADRs [8]. The existed longitudinal studies are limited by investigating an individual bDMARD, rarely describe the safety in a single sitting, and didn’t highlight the time to develop each ADR. It is hypothesized that all bDMARDs are equally safe. However, more longitudinal studies are needed to confirm this hypothesis. Therefore, this study aimed to investigate the hazard of using different IV originator bDMARDs in comparison to each other and to assess the survival of this hazard in RA patients with or without csDMARDs in Saudi Arabia.
Materials and Methods
The study is a continuation of a previous study that assessed the prevalence of ADRs, as defined by the WHO, of IV bDMARDs in RA patients [5]. The study was a retrospective cohort of adult patients diagnosed with RA and undergoing IV bDMARD therapy. Within each bDMARD, a cohort of participants with identified ADRs was compared to a cohort of participants with no identified ADRs. The study is observational with no planned intervention and was conducted in King Saud University Medical City hospital in Riyadh, Saudi Arabia. Data extraction was between January 2019 and March 2020. The study is guided by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for cohort study designs (STROBE) checklist for cohort study designs [10].
Patients in this study were considered eligible if they meet all of the following criteria: the presence of a confirmed RA diagnosis according to the 2010 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria [11]. Aged ≥ 18 years and receiving ≥ 1 dose of IV originator bDMARDs available at the study setting (ABA, IFX, TCZ, or RTX) with or without csDMARDs.
For inclusion in the study, participants were screened for eligibility by reviewing the electronic files and medical daycare unit logbook. The medical file numbers of all adult patients (more than 18 years of age) and receiving IV bDMARD therapy for RA from the start of the electronic medical records in January 2015 until January 2020 were identified and reviewed. Each file number was reviewed individually to confirm that patient received one of the bDMARDs for confirmed RA diagnosis. Then all visits and labs of each patient were explored from the time of first receiving bDMARDs till the discontinuation or change of therapy. All abnormal labs and events with corresponding dates were recorded. For exclusion of the study, any file number for the patient receiving one of the bDMARDs for diseases other than RA or aged less than 18 years were excluded.
By the standard of care, patients were monitored for efficacy and ADRs during infusion time and every three to six months. A list of commonly reported ADRs of IV bDMARDs being studied was created from the existing literature and was set as a database to evaluate its presence in correspondence to bDMARD therapy [12-16]. Additional ADRs were observed in our sample and also assessed for causality by three authors (Hawazin S. Alhazzani, Lamia A. Alzamel, Reem S. Tashkandi) using the Naranjo scale and then checked and documented by two senior authors (Shiekha S. AlAujan, Haya M. Almalag) [17]. The patients’ files were reviewed for the development of any ADR, its type, and the time until it developed after the administration of IV bDMARDs under study. The following data were collected: patient demographics, and IV bDMARD information, such as dose, number of doses the ADR occurred with, previous medications, concomitant medications, frequency, date of administration, date of ADR development, date of the last follow-up, mortality, laboratory information (including seropositivity as indicated by either rheumatoid factor positive, anticitrullinated peptide positive or both) and the Charlson comorbidity score [18]. The number of bDMARDs or csDMARDs received before IV bDMARDs and the reason for the discontinuation of previous medication were also reported. Any document in the patient file for discontinuation due to failure of therapy was documented as “therapy failure” in the study.
Given the effect size and differences of serious infections reported in Gron, et al., for TCZ versus RTX of 0.22 [1]. With an estimated 5% margin of error and 95% confidence level, the calculated sample size is 264. However, it was intended to include all participants taking IV bDMARDs for the last five years.
Data were coded, entered, and analyzed using the Statistical Package Software for Social Sciences (SPSS©) version 26 and R version 4.0.3. Each patient was assigned a unique study number with no identifiers collected to ensure patient confidentiality. The mean and Standard Deviation (SD) were presented for normally distributed data; skewed data were expressed as median and 25th and 75th Interquartile Ranges (IQR). Only ADRs with a Naranjo score ≥ 1 were included; this score is interpreted as possible (1-4), probable (5-8), and definite (≥ 9). The Kaplan-Meier survival analysis and the Cox proportional-hazards model were employed to investigate the free survival of ADRs, allergic reaction, infection, and serious ADRs according to the type of IV bDMARDs. According to the WHO, serious ADR is defined as “any event that Is fatal, life-threatening, permanently/significantly disabling, requires or prolongs hospitalization, causes a congenital anomaly or requires intervention to prevent permanent impairment or damage” [5]. ABA was chosen as a reference as it was the most recent IV bDMARD added to the hospital formulary. The crude associations between exposure and survival were evaluated via. the Kaplan-Meier analysis. The Cox proportional-hazards model was used to estimate the Hazard Ratios (HRs) and 95% Confidence Intervals (CIs) controlled by confounders using only completed cases between the two cohorts for each DMARDs. Significant confounders were controlled in the analysis. The frequency of using each bDMARD was not added to the regression model as it was reflected by time in days to avoid collinearity. We attempted to perform the competing risk approach as the occurrence of death events would prevent an individual from experiencing the study outcomes used in the survival analysis. Nevertheless, no subject in the study died during the follow-up period. Therefore, this approach was not employed. No violations of the proportional-hazards assumption were noted.
For ethical consideration no informed consent was obtained as the study was retrospective; however, no patient identifiers were collected to ensure patient confidentiality. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of King Saud University Medical City (Approval project number E-18-3621), and only involved researchers with ethical standards certification had access to the data in a locked computer.
Results
Of the 3000 patient files reviewed, 129 were included in the study. The majority of the patients were female (87.6%), with a mean age of 54 (± 13) years and a mean disease duration of 11.64 (± 7.4) years (Table 1). Most of the patients were also seropositive (76.7%), with an average of 2.06 (± 1.19) previously received bDMARDs. The median followup duration was 847.00 days (IQR: 741.8, 917.6), and a total of 1963 doses were administered. Patients received TCZ (n=50, 38.8%), RTX (n=50, 38.8%), ABA (n=18, 14.0%) and IFX (n=11, 8.5%). Table 1 presents the demographic characteristics of the study participants, addition complete baseline demographic, medication, and comorbidities stratified by bDMARD and p-value of difference is available in appendix A.
| Baseline characteristics | Total (n=129) |
|---|---|
| Age Years, mean (SD*) | 54 (13) |
| Gender, n (%) Female | 113 (87.6%) |
| Disease duration Years, mean (SD) | 11.64 (7.4) |
| Seropositive to either RF†, anti-CCP‡ or both, n (%) | 99 (76.7%) |
| Number of previous bDMARDs§ received, mean (SD) | 2.06 (1.2) |
| Number of bDMARDs in response to other RA** medications, mean (SD) | 2.03 (1.2) |
| Current intravenous bDMARDs, n (%) | |
| Abatacept | 18 (14.0%) |
| Tocilizumab | 50 (38.8%) |
| Infliximab | 11 (8.5%) |
| Rituximab | 50 (38.8%) |
| Concomitant RA medications, n (%) | |
| Methotrexate | 72 (55.8%) |
| Oral glucocorticoid | 33 (25.6%) |
| Other DMARDs | 29 (22.5%) |
| * Standard Deviation; †Rheumatoid Factor; ‡Anticitrullinated Peptide; § Biologic Disease-Modifying Antirheumatic Drugs; **Rheumatoid Arthritis | |
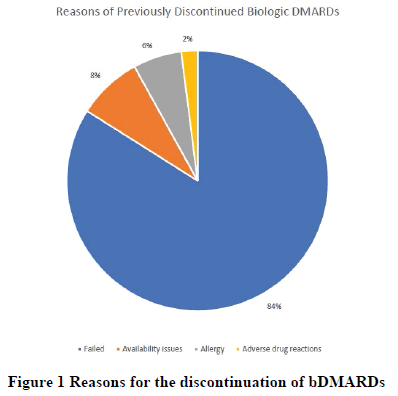
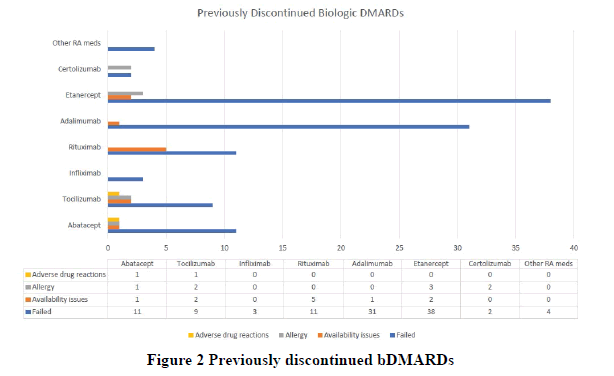
Age, gender, Charlson index, baseline infection, fibromyalgia, and the use of proton pump inhibitors and angiotensinconverting enzyme inhibitors were significant on univariate analysis and controlled in the regression analysis (univariate analysis is available in appendix Table A2). The most common reasons for the discontinuation of previous bDMARDs were the failure of therapy, followed by availability issues and allergy, whereas the least common reason for discontinuation was ADR (Figure 1). Among the study participants, etanercept was the most commonly discontinued bDMARD, followed by adalimumab (Figure 2).
Majority of the patients experienced at least one ADR with a Naranjo scale score >1 (possible) (n=100, 77.5%), followed by a serious ADR (n=69, 53.5%), then an infectious ADR (n=56, 43.4%). The least commonly experienced ADR was the allergic reaction (n=7, 5.4%). It took a median of 188 days (IQR 235.3-334.3) and 391 days (IQR 370.2- 488.0) before the development of the first episode of any ADR and serious ADR, respectively. The median time before the development of the first infectious ADR was 456 days (IQR 472.4-621.1). Finally, the first allergic reaction event took the longest to occur, with a median time of 758 days (IQR 710.4-888.4). Table 2 presents an analysis of the ADRs experienced while on bDMARDs.
| Characteristics | Total (n=129) |
|---|---|
| Any ADR*, n (%) | 100 (77.5%) |
| Allergic reaction, n (%) | 7 (5.4%) |
| Infection, n (%) | 56 (43.4%) |
| Serious ADR, n (%) | 69 (53.5%) |
| Time to first ADR, median days (IQR) | 188 (235.3-334.3) |
| Time to first infectious ADR, median days (IQR†) | 456 (472.4-621.1) |
| Time to first serious ADR, median days (IQR) | 391 (370.2-488.0) |
| *Adverse Drug Reaction; † Interquartile Range | |
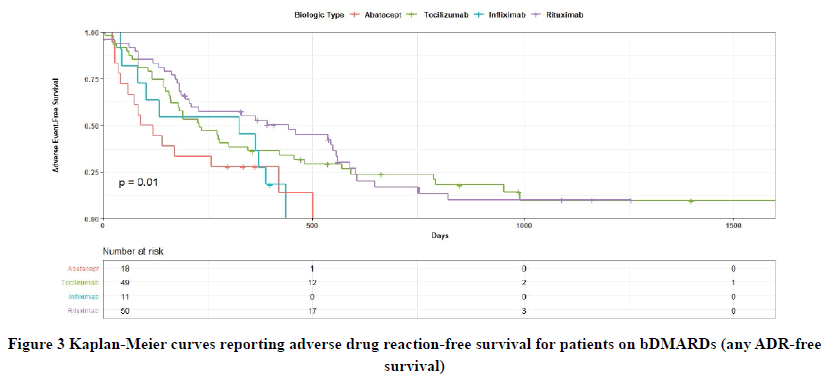
For ADR-free survival a total of four files had missing information; hazard analysis was conducted only on complete cases. The Kaplan-Meier analysis and log-rank test demonstrated a significant difference in all kinds of ADR-free survival among the included bDMARDs (p=0.01) (Figure 3) with ABA as a reference group. The entire follow-up period was 1906 days, but all ADRs were experienced within 1500 days of follow-up. In the univariate HR analysis (Table 3), RTX was associated with lower ADR of all kinds in 1500 days of follow-up compared with ABA (HR=0.39, 95% CI: 0.21-0.72, p=0.003). This was followed by TCZ which was also associated with less ADR of all kinds compared with ABA in 1500 days of follow-up (HR=0.45, 95% CI: 0.2-0.84, p=0.012). IFX also exhibited a trend toward lower ADRs compared with ABA in 1500 days of follow-up, but this was statistically insignificant (HR=0.77, 95% CI: 0.35-1.73, p=0.5). Additionally, after adjusting for age, gender disease duration, and confounding variables in the multivariate HR analysis, RTX was still associated with lower ADR in 1500 days of follow-up compared with ABA (HR=0.32, 95% CI: 0.17-0.61, p<0.001) followed by TCZ (HR=0.37, 95% CI: 0.20-0.71, p=0.002). The results involving IFX remained insignificant (HR=0.72, 95% CI: 0.32-1.63, p=0.4). Table 3 presents the results of the univariate and multivariate HR analyses. With regards to the survival of allergic reaction by bDMARD was not calculated as the sample was too small to detect change (n=7, 5.4%).
| Characteristic | Any ADR* | Infection | Serious ADR | |||
|---|---|---|---|---|---|---|
| HR † (95% CI ‡) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Univariate hazard ratio | ||||||
| Abatacept | Reference | Reference | Reference | |||
| Tocilizumab | 0.45 (0.24-0.84) | 0.012*** | 0.54 (0.25-1.17) | 0.12 | 0.66 (0.33-1.32) | 0.2 |
| Infliximab | 0.77 (0.35-1.73) | 0.5 | 0.86 (0.29- 2.55) | 0.8 | 0.60 (0.22-1.62) | 0.3 |
| Rituximab | 0.39 (0.21-0.72) | 0.003*** | 0.53 (0.24-1.17) | 0.12 | 0.48 (0.23-1.00) | 0.051 |
| Multivariate hazard ratio | ||||||
| Abatacept | Reference | Reference | Reference | |||
| Tocilizumab | 0.41 (0.21-0.81) | 0.01*** | 0.55 (0.23-1.28) | 0.20 | 0.65 (0.31-1.34) | 0.20 |
| Infliximab | 0.57 (0.23-1.43) | 0.20 | 0.96 (0.26-3.49) | >0.90 | 0.39 (0.12-1.23) | 0.11 |
| Rituximab | 0.29 (0.15-0.56) | <0.001*** | 0.52 (0.23-1.17) | 0.11 | 0.48 (0.21-1.08) | 0.08 |
| Age | 0.99 (0.97-1.01) | 0.14 | 1.00 (0.97-1.02) | >0.90 | 0.98 (0.96-1.00) | 0.11 |
| Gender (female to male) | 2.12 (1.06-4.25) | 0.03 | 0.65 (0.25-1.70) | 0.40 | 1.61 (0.68-3.80) | 0.30 |
| Disease duration | 1.02 (0.99-1.05) | 0.20 | 1.00 (0.97-1.04) | 0.80 | 1.02 (0.98-1.06) | 0.30 |
| Charlson index score | 1.15 (0.95-1.39) | 0.14 | 1.01 (0.80-1.26) | >0.90 | 1.09 (0.88-1.35) | 0.40 |
| ACEIs § | 0.79 (0.37-1.70) | 0.60 | 1.78 (0.69-4.60) | 0.20 | 0.75 (0.28-2.02) | 0.60 |
| PPIs** | 1.46 (0.92-2.32) | 0.11 | 1.24 (0.66-2.34) | 0.50 | 1.29 (0.75-2.23) | 0.40 |
| Fibromyalgia | 1.73 (0.91-3.32) | 0.10 | 1.72 (0.76-3.91) | 0.20 | 1.83 (0.90-3.72) | 0.09 |
| Infections | 1.07 (0.57-2.01) | 0.80 | 0.61 (0.23-1.58) | 0.30 | 0.90 (0.43-1.87) | 0.80 |
| *Adverse Drug Reaction; †Hazard Ratio; ‡Confidence Interval; §Angiotensin Converting Enzyme Inhibitors; **Proton Pump Inhibitors | ||||||
| *** Significant according to a significance level of p<0.05 | ||||||
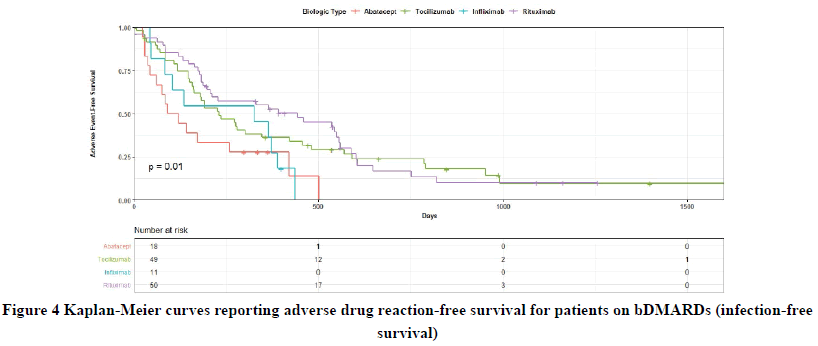
No significant difference in the infection-free survival was observed between the bDMARDs and ABA in 1500 days of follow-up (p=0.32) (Figure 4). Although RTX and TCZ exhibited a trend towards lower infection compared with ABA in 1500 days of follow-up, this was not significant in the univariate analysis (HR=0.53, 95% CI: 0.24-1.17, p=0.12; HR=0.54, 95% CI: 0.25-1.17, p=0.12; respectively). The same was true after conducting the multivariate analysis. IFX was also not associated with a significant difference in infection-free survival in 1500 days of follow-up both in the univariate and multivariate HR analyses, as can be seen from Table 3.
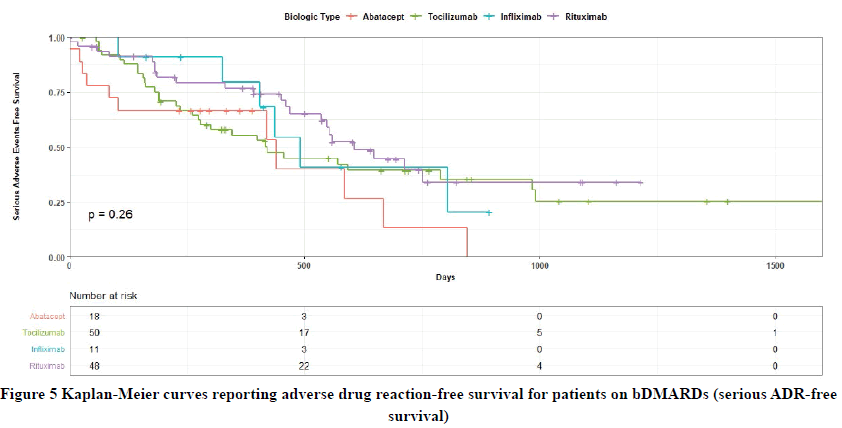
Although no significant differences were observed in serious ADR-free survival between the bDMARDs and ABA in 1500 days of follow-up (p=0.26) (Figure 5), RTX showed less serious ADRs in the univariate HR analysis in 1500 days of follow-up compared with ABA, and this was approaching the significance (HR=0.48, 95% CI: 0.23-1.00, p=0.051). The remaining bDMARDs did not demonstrate significant results compared with ABA about serious ADRfree survival in both univariate and multivariate HR analyses as presented in Table 3.
Discussion
The use of bDMARDs is essential for patients who have undergone unsuccessful csDMARD therapies. However, safety issues are a major concern while considering IV bDMARD therapy. Data comparing different IV bDMARDs in head-to-head trails in the same setting are rare. Head-to-head studies are essential for making well-informed therapeutic decisions in the treatment of RA patients. Moreover, studies reporting time to develop ADR using survival analysis were not performed previously. In this study, we compared the ADRs of four different IV bDMARDs in a longitudinal single-centered study. We reported the hazards of using different IV bDMARDs and the time until the development of ADRs. Moreover, we determined the reasons for the discontinuation of previous bDMARDs along with the most previously discontinued bDMARDs. Reasons for variation in the number of used bDMARDs were based on the indication of each bDMARD, failure of therapy, and the availability of the bDMARD in the study setting.
Our study demonstrated that RTX and TCZ were associated with lower ADRs of all kinds in 1500 days of follow-up compared with ABA. Several studies have already attempted to evaluate the safety of bDMARDs in RA patients. Given the lack of head-to-head studies comparing IV bDMARDs, Gartlehner and colleagues conducted a metaanalysis of the safety and efficacy between bDMARDs in RA using data from placebo-controlled trials [8]. The results revealed that infusion-related allergic reactions were the most frequently reported ADRs for ABA, RTX, and IFX. Although they conducted indirect comparisons between IFX and other anti-tumour necrosis factors (Anti-TNF) drugs and between IFX and anakinra, the data was not sufficient to make an indirect comparison between ABA and rituximab.
In our study, no statistically significant difference was observed in infection-free survival in 1500 days of follow-up between the included IV bDMARDs with ABA as the reference comparator. These findings are consistent with a longitudinal comparative study performed by Curtis, et al. in 2016 [19]. They evaluated the risk of herpes zoster and herpes simplex virus infections in RA patients treated with bDMARDs. They found no significant difference, except for tofacitinib, in the hazard rates per 100 patient-years between the included bDMARDs (TCZ, IFX, and RTA) and ABA.
In 2019, Gron and colleagues also studied the risk of serious infections in RA patients treated with ABA, TCZ, and RTA [20]. Their study was conducted simultaneously in both Denmark and Sweden using an observational cohort design. They found that serious infections in 12 months exhibited a trend towards lower Relative Risk (RR) for TCZ versus RTX (RR 0.78 (95% CI: 0.61, 1.01)) and ABA versus RTX (RR 0.88 (95% CI: 0.69, 1.12)). They also found a higher RR when comparing ABA versus TCZ (RR 1.13 (95% CI: 0.91, 1.42)). However, their findings were statistically insignificant as the 95% CI crossed 1.
In our study, we reported ADRs that were also commonly reported in the literature. Some studies have also addressed other ADRs aside from the ones we reported. In 2018, Jin and colleagues investigated the risk of cardiovascular ADRs in RA patients following the initiation of ABA compared with TNF inhibitors (including infliximab) in a cohort study [21]. They found that ABA was associated with a reduced risk of cardiovascular disease when compared with TNF inhibitors (HR 0.79 (95% CI: 0.67, 0.92)), and this remained significant even after the adjustment for baseline cardiovascular disease.
In our cohort, we also demonstrated that failure of therapy was the most common reason behind the premature discontinuation of bDMARDs. Etanercept was the most frequently discontinued bDMARD, followed by adalimumab. However, this finding contradicts previous reports. In 2013, Arora and colleagues conducted a systematic review to assess the drug survival of bDMARD therapies in RA patients. After 60 months of follow-up, etanercept was found to have the lowest discontinuation rate compared with adalimumab, whereas IFX demonstrated the highest discontinuation rate [22]. Their findings were supported by another meta-analysis conducted by Souto and colleagues using data from healthcare databases and drug registries [23]. They found that the discontinuation rate of etanercept was significantly lower at 3 and 4 years compared with IFX and adalimumab. The discrepancy in this outcome might be because the etanercept was not stocked in our hospital in the last few years. Other secondary factors which we did not control might have also contributed to the discontinuation of etanercept in our study. The authors of the previously mentioned study discussed how disease duration, female gender, and concomitant use of csDMARDs are predictors of the discontinuation of TNF inhibitors. Moreover, one can speculate that the difference in the population characteristics of Saudi patients and genetic predispositions might have played a role in this discrepancy. The pharmacogenetics of etanercept in RA patients was evaluated previously, and genetic polymorphism was found to be a predictor of treatment safety and efficacy [24].
The current study has some limitations. First, this was a single-centre study limited by a small sample size and a small number of participants in some groups; this might have affected the precision and detecting significance in our findings, particularly the HR of allergic reactions. Secondly, due to the retrospective nature of the study, some participants had missing data, and a complete case analysis was conducted to overcome the effect of missing data. However, the current study has a longitudinal study design, which captures real-world data concerning the safety of four originator IV bDMARDs in one study. We also evaluated the causality of developed ADR to bDMARDs using the Naranjo scale. Future studies can further assess the safety of IV bDMARDs in a larger sample of patients and a multi-center setting. An assessment that accounts for the effect of the Saudi population characteristics on treatment response and toxicity is also required.
Conclusion
In this retrospective study, there is significantly less hazard of developing ADRs while using IV bDMARDs, namely, TCZ and RTA, compared with ABA therapy in RA patients with or without csDMARDs. Insights on the safety of IV bDMARDs in comparison with each other from a real-life setting might be of great use for daily clinical practise to treat people with RA.
Declarations
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
The project is funded by Researchers Supporting Project Number RSP-2020/209, King Saud University, Riyadh, Saudi Arabia.
References
- Singh, Jasvinder A., et al. "2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis." Arthritis & Rheumatology, Vol. 68, No. 1, 2016, pp. 1-26.
- Felis-Giemza, Anna. "New era of treatment with biologics in rheumatology-Is it time to shift paradigms in treatment with biologics?" Reumatologia, Vol. 57, No. 5, 2019, pp. 255-56.
- Rein, Philipp, and Ruediger B. Mueller. "Treatment with biologicals in rheumatoid arthritis: An overview." Rheumatology and Therapy, Vol. 4, No. 2, 2017, pp. 247-61.
- de Camargo, Mayara Costa, et al. "Adverse events in patients with rheumatoid arthritis and psoriatic arthritis receiving long-term biological agents in a real-life setting." Frontiers in Pharmacology, Vol. 10, 2019, p. 965.
- World Health Organization. "Safety of medicines: a guide to detecting and reporting adverse drug reactions: why health professionals need to take action." No. WHO/EDM/QSM/2002.2. World Health Organization, 2002.
Google Scholar Crossref
- Treharne, G. J., et al. "Polypharmacy among people with rheumatoid arthritis: The role of age, disease duration and comorbidity." Musculoskeletal Care, Vol. 5, No. 4, 2007, pp. 175-90.
- Huynh, Tuan Khai, et al. "Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis." Patient Preference and Adherence, Vol. 8, 2014, pp. 93-99.
- Gartlehner, Gerald, et al. "The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: A systematic review and metaanalysis." The Journal of Rheumatology, Vol. 33, No. 12, 2006, pp. 2398-408.
Google Scholar Crossref
- Sepriano, Alexandre, et al. "Safety of synthetic and biological DMARDs: A systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis." Annals of the Rheumatic Diseases, Vol. 79, No. 6, 2020, pp. 760-70.
- Von Elm, Erik, et al. "The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies." Bulletin of the World Health Organization, Vol. 85, 2007, pp. 867-72.
- Aletaha, Daniel, et al. "2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative." Arthritis & Rheumatism, Vol. 62, No. 9, 2010, pp. 2569-81.
- Van Vollenhoven, Ronald F., et al. "Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients." Annals of the Rheumatic Diseases, Vol. 72, No. 9, 2013, pp. 1496-502.
- Weisman, Michael H., et al. "Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis." The Journal of Rheumatology, Vol. 33, No. 11, 2006, pp. 2162-66.
Google Scholar Crossref
- Westhovens, Rene, et al. "Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors." Annals of the Rheumatic Diseases, Vol. 68, No. 12, 2009, pp. 1870-77.
- Downey, Colum. "Serious infection during etanercept, infliximab and adalimumab therapy for rheumatoid arthritis: A literature review." International Journal of Rheumatic Diseases, Vol. 19, No. 6, 2016, pp. 536-50.
- Singh, Jasvinder A., Saba Beg, and Maria Angeles Lopez-Olivo. "Tocilizumab for rheumatoid arthritis: A Cochrane systematic review." The Journal of Rheumatology, Vol. 38, No. 1, 2011, pp. 10-20.
- Naranjo, Claudio A., et al. "A method for estimating the probability of adverse drug reactions." Clinical Pharmacology & Therapeutics, Vol. 30, No. 2, 1981, pp. 239-45.
- Charlson, Mary E., et al. "A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation." Journal of Chronic Diseases, Vol. 40, No. 5, 1987, pp. 373-83.
- Curtis, Jeffrey R., et al. "Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis." Annals of the Rheumatic Diseases, Vol. 75, No. 10, 2016, pp. 1843-47.
- Gron, Kathrine Lederballe, et al. "Risk of serious infections in patients with rheumatoid arthritis treated in routine care with abatacept, rituximab and tocilizumab in Denmark and Sweden." Annals of the Rheumatic Diseases, Vol. 78, No. 3, 2019, pp. 320-27.
- Jin, Yinzhu, et al. "Cardiovascular (CV) risk after initiation of abatacept versus TNF inhibitors in rheumatoid arthritis patients with and without baseline CV disease." The Journal of Rheumatology, Vol. 45, No. 9, 2018, pp. 1240-48.
- Arora, Anamika, et al. "Long-term drug survival of TNF inhibitor therapy in RA patients: A systematic review of European national drug registers." International Journal of Rheumatology, Vol. 2013, 2013.
- Souto, Alejandro, José Ramón Maneiro, and Juan J. Gomez-Reino. "Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: A systematic review and meta-analysis of drug registries and health care databases." Rheumatology, Vol. 55, No. 3, 2016, pp. 523-34.
- Danila, Maria I., Laura B. Hughes, and S. Louis Bridges. "Pharmacogenetics of etanercept in rheumatoid arthritis." Pharmacogenomics, Vol. 9, No. 8, 2008, pp. 1011-15.