Research - International Journal of Medical Research & Health Sciences ( 2020) Volume 9, Issue 12
Designs, synthesis, structural elucidation and antimicrobial evaluation of various derivatives of 2-mercaptobenzimidazole as possible antimicrobial agents
Rand Al-kazweeny1, Zuhair A Muhi-eldeen1, Elham Al-kaissi2*, Sadeq Al-tameemi1, Sumia S Tayeh1 and Jaafar Al-hussenini12Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Petra, Amman, Jordan
Elham Al-kaissi, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Petra, Amman, Jordan, Tel: 962 6 579 9555, Email: ealkaissi@uop.edu.jo
Received: 07-Oct-2020 Accepted Date: Dec 21, 2020 ; Published: 28-Dec-2020
Abstract
Objective: Derivatives of 2-Mercaptobenzimidazole (2-MBI) are very significant heterocyclic compounds. 2-MBI and its derivatives have been showing different biological activates, the most significant activity exhibiting the antimicrobial activity. The aim of the project includes the synthesis of new derivatives of 2-MBI. Methods: Formation of 2-(Phenethylthio)-1H-benzo[d] imidazole (ZR-1) by alkylation, thioester S-(1H-benzo[d]imidazol-2-yl) O-ethyl carbonothioate (ZRJ2), S-(1H-benzo[d]imidazol-2-yl) O-isobutyl carbonothioate (ZR-4), and S-(1H-benzo[d] imidazol-2-yl) O-methyl carbonothioate (ZR-8), the reaction involves thio acetic derivative 2-((1H-benzo[d]imidazol2-yl) thio) acetic acid (ZR-3) with oxalyl chloride to generate acyl derivatives to this added cyclic amines to yield compounds, amid derivatives 2-((1H-benzo[d]imidazol-2-yl) thio)-1-(2, 6-dimethylpiperidin-1-yl) ethan-1-one (ZR-5), 2-((1H-benzo[d]imidazol-2-yl) thio)-1-(pyrrolidin-1-yl) ethan-1-one (ZR-6), and 2-((1H-benzo[d]imidazol2-yl) thio)-1-(4-methylpiperazin-1-yl) ethan-1-one (ZR-7). Results: Structure of newly synthesized compounds have been verified through FT-IR, DSC, Elemental analysis, 1H-NMR, 13C-NMR, and Molecular docking, it indicates one of these compound (ZR-5) has great affinity (-8.70) than our stander (trimethoprim -7.91Kcal/ mol. into DHFR). An antimicrobial activity was evaluated by agar diffusion methods and broth dilution test against Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 8739), Pseudomonas aeruginosa (ATCC 9027), Bacillus subtilis (ATCC 6633) and Candida albicans (ATCC 10231). The Minimum Inhibitory Concentrations (MIC) and the Minimum Bactericidal Concentration (MBC) were determined and compared with ciprofloxacin (85.51%), trimethoprim and fluconazole (99.9%) as standard positive control drugs, Conclusion: Compound (ZR-8) showed excellent antifungal activity and good antibacterial activity compared to ciprofloxacin and trimethoprim. The results promoted the interest to do more structural modifications to enhance the antimicrobial activity and their antimicrobial selectivity
Keywords
2-mercaptobenzimidazole, Alkylation, Amidation, Acyl formation
Introduction
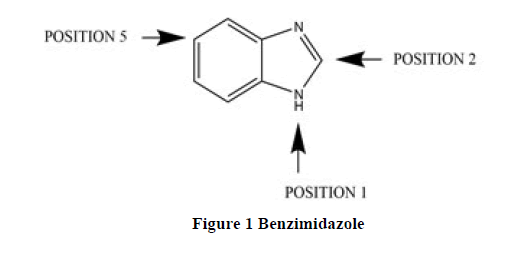
Benzimidazole is an important pharmacophore and a privileged structure in medicinal chemistry, is a heterocyclic aromatic organic compound (Figure 1) [1]. Benzimidazole is bicyclic in nature which consists of the fusion of benzene and imidazole [2]. Derivatives of benzimidazole were synthesized with the substitution of Fluorine, Propylene, tetrahydroquinoline which lead to compounds with increased stability, bioavailability and significant biological activity [3].
Benzimidazole containing compounds have different medical and biological activities, such as antitumor, antibacterial, antifungal, antiviral, anticonvulsant, antidepressant analgesic, anti-inflammatory, antidiabetic properties [4-11]. Almost all benzimidazole derivatives with their two ring systems afford different functional substituents and this leads to essential modification of the metabolic and pharmacokinetic properties physico-chemical, of these drugs. In the past few decades, benzimidazole and its derivatives have received much attention because of their chemotherapeutic values, Antioxidant Activity Oxygen-derived, Antimicrobial and Antibacterial Activity Benzimidazole shows their antibacterial activity by inhibiting the bacterial nucleic acid and proteins synthesis [12]. Benzimidazole and imidazole drugs have wide range in remedying different dispositions in clinical medicine [13]. Imidazole nucleus forms the main structure of some well-known components of human body, that is, the amino acid histidine, Vit-B12, a component of DNA base structure and purines, histamine, and biotin [14]. There are drugs of Imidazole have a broader scope in treatment of different dispositions in clinical medicine [14]. It is also present in the structure of many natural or synthetic drugs molecules. 2-mercaptobenzimidazole derivatives, one of the most important derivatives of benzimidazole exhibited a wide variety of interesting biological activities such as antimicrobial, antihistamine, neutropic, and analgesic activities [15-18].
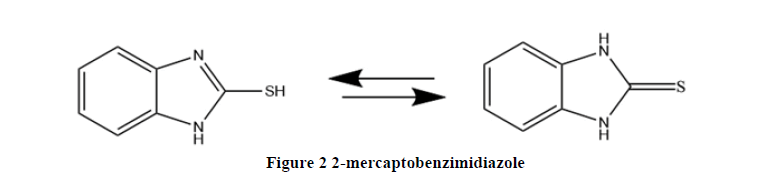
2-Mercaptobenzimidazole derived from benzimidazole with thiol group in the 2-position (Figure 2). It possesses other chemical names such as, o-phenylen thiourea, benzimidazol-2-thion with formula of C7H6N2S [19]. Some characteristic of 2-mercaptobenzimid- azole are containing of thioamide group (-N-C=S), therefore it is considered one of thioamide compounds for its ability to react under special condition to give derivatives having substituent at either nitrogen or sulfur atoms, 2-mercaptobenzimidiazole possess the form dimer, because it has (C=S) group, this preferable product is the dimer, it is known to exist in two tautomerism forms, the thiol and thion from as represented below [20, 21].
In reviewing most of antimicrobial drugs or possible investigated antimicrobial agent we envision the addition of groups that changes the nature of the thiol group in 2-mercapto benzimidazole. Thiol conversion in to various derivatives reflex new compounds with different interaction with the site of action. Cyclic amine may exist in different conformation that generates different activity. The hydrocarbons (ethyl, methyl, isobutyl) represent various in lipophilicity. COOH (R) hydrophilic in nature and could be converted in to various derivatives. Unique changes in groups are expected to generate effective antibacterial and antifungal agent.
Materials and Methods
Chemicals
The following chemicals was used: 2-mercaptobenzimidazole (98%), Piperidine 99%, 2,6 Dimethyl piperidine, Pyrrolidine, Methyl chloroformate, Ethyl chloroformate, Isobutyl chloroformate, Oxalyl chloride 99%, 2-Chloroethanol 99%, Chloroacetic acid 99%, 2-Bromoehyl benzene 98% all of them (Sigma Aldrich USA), Magnesium sulfate anhydrous (Lonover, UK), Potassium bromide (KBr) (Scharlau, Spain), Potassium carbonate anhydrous (K2CO3) (Gainland Chemical Company (GCC), UK), Potassium hydroxide (KOH) (Lonover, UK), N, N-Dimethylformamide (DMF) (AZ chem, Canada), Absolute ethanol 99.9% (Super chem, India), Dichloromethane 99.8% (Medex, UK), Acetone 99% (Scharlau, Spain). Chloroform extra pure (TEDIA, USA), Distilled water (Ultra, Joran).
Instrumentation
Analytical balance with a precision 0.01 mg (Phoenix instrument, USA), hot plate with magnetic stirrer (IKAMAG, India), Rotary evaporator (Roker 600, Germany), Buchner Funnel Pump (Vacuubrand, Germany). Melting point was determined on Melting Point Apparatus (GallenKamp, Germany). The structures of the synthesized compounds were confirmed by Differential Scanning Calorimetry (DSC) (Mettler Toledo, USA), Nuclear Magnetic Resonance (NMR) 300 MHZ (Varian 300 MHZ, Bruker, USA), elemental analysis was performed for C, H, N using Euro EA elemental analyzer, the results obtained had a maximum deviation of (± 0.4) from the theoretical value, which is considered within the acceptable variation range in results (± 4%) (Euro Vector, Italy). This variation range is set according to the accuracy of Euro EA elemental analyzer device. FT-IR (Perkin Elmer 7600 Germany), Infra-Red (IR). DSC thermogram measurement was carried out by using the DSC 1 stare system v.11.ox. ChemBioDraw (Massachusetts, U.S.A) was used in the drawing of our schemes.
Docking
The Dihydrofolate Reductase (DHFR) protein target was selected from RCSB databank. The DHFR complex with inhibitor with PDB id 3GHW was downloaded for study. The NDPH is an important cofactor in DHFR activity. Thus the enzyme complex with NADPH was extracted. The complex was then used for minimizing the structure. The GROMACS, Linux based software was used for DHFR and NADPH complex minimization. The DHFR and NADPH complex was used as a docking target enzyme. The ligand structure was constructed using ChemDraw 2D. Then it was used to get three-dimensional structures of ligand using Chem3D. Also the ligand was energy minimized using MM2 force field present in Chem3D. Then, ligands and enzyme were prepared for docking using AutoDock Tools. The grid for docking was prepared using the inhibitor site and used it as a template. The Lamarkin GA was used to perform the docking. The docking was performed for 10 conformations and done used using AutoDock 4.2. The results were then analyzed using PyMOL and Chimera visualization software.
Selected Test Microorganisms
Staphylococcus aureus (S. aureus ATCC 6538), Bacillus subtilis (B. subtilis ATCC 6633), Pseudomonas aeruginosa (P. aeruginosa ATCC 9027), Escherichia coli (E. coli ATCC 8739), Candida albicans (C. albicans ATCC 10231), all these pure cultures of bacterial and fungal strains were obtained from Dar Al-Dawa (Na’ur, Jordan).
Culture Media
Muller Hinton Broth (MHB) (Mastgrp Ltd, UK), Mueller Hinton Agar (MHA) (Mastgrp Ltd, UK)/ (Himedia, India), Sabourauds Dextrose Broth (SDB) (Himedia, India), Sabourauds Dextrose Agar (SDA) (Mastgrp Ltd, UK).
Evaluation of Antimicrobial Activity
The antimicrobial activity of the newly synthesized compounds of different derivatives of 2-mercaptobenzimidazole (ZR1-ZR8) were evaluated by agar diffusion method against Gram-positive bacteria, Gram-negative bacteria and fungi, using S. aureus (ATCC 6538), E. coli (ATCC 8739), P. aeruginosa (ATCC 9027), B. subtilis (ATCC 6633), and C. albicans (ATCC 10231). Mueller Hinton agar or Sabourauds dextrose agar was used in a petri dish and overwhelm its surface with 0.1 ml (1 × 106 CFU/ml) inoculum of the required microorganism, Mueller Hinton agar is used for bacteria, while Sabourauds dextrose agar for fungi [22]. Then wells were cut and filled with 0.2 ml of the synthesized compounds (ZR1-ZR8) in concentrations of 500 μg/ml. The plates were left at room temperature for 30 min, and then the plates were incubated at 37°C for 24 h for bacteria, and at 25°C for 48 h for fungi. The zone of inhibition was determined by measuring the diameter of the zone formed around each wells and the results were compared with a positive control (100 μg/ml ciprofloxacin and trimethoprim 200 μg/ml dissolved in DMSO for bacteria, 500 μg/ml miconazole dissolved in DMSO for fungi), and negative control (DMSO alone) was included also [23].
The Minimum Inhibitory Concentration (MIC) against both bacteria and fungi and the Minimum Bactericidal or Fungicidal Concentration (MBC/MFC) was determined by microplate broth dilution technique. Briefly, MIC, MBC
or MFC were determined by the broth twofold micro dilution method in Mueller Hinton broth or Sabourauds dextrose broth, according to a modification of the procedure reported by NCCLS, 2012 [24]. Compounds (ZR-1 to ZR-8) were dissolved in DMSO (1 ml) to obtain concentration of 500 μg/ml as stock solutions. The serial dilution method was used to obtain (250 to 0.488) μg/ml concentrations. Mueller-Hinton broth was used as medium for bacterial growth, 100 μl of each concentration were distributed in 96-well plates with the exception of those wells acting as growth control (contain microorganisms and culture media) and positive control (contain microorganisms and standard control antibiotic), 20 μl of the standard microorganism’s inoculum (equivalent to 1 × 106 CFU) were added to each well except the negative control well, the microplates were covered and sealed with parafilm and incubated at 37°C for 18-24 h for bacteria and at 25°C for 28 h for fungi. Resazorin indicator solution in 50 μl was added to each well, the microplates were incubated as mentioned before for 30 min and reported, violet color of the contain of the well indicated no bacterial growth, if the color change to pink it indicated microbial growth.
To determine the MBC or MFC, a loop full with 10 μl was removed from the MIC dilution well and from the preceding dilutions, from the negative and positive control wells, all of them were inoculated into MHA or SDA plates and incubated at 37°C for bacteria or 25°C for fungi for 24 h or 48 h respectively, the lower concentration which gave no growth was the required MBC or MFC, and were compared to the MBC or MFC of the positive antimicrobial control [25,26]. All determinations were carried out in triplicate.
Statistical Analysis
The analysis was carried by using the statistical packages for social science SPSS version 21 for student’s t-test. Values are presented as mean ± SD.
Synthesis
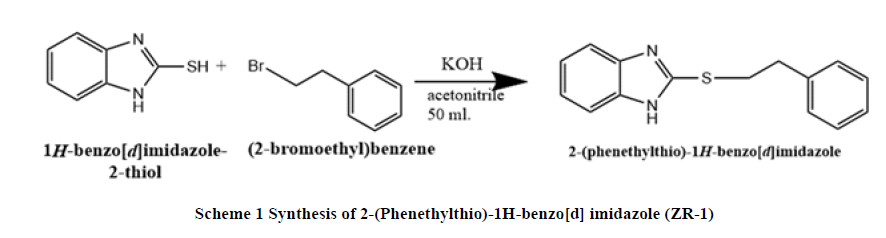
Synthesis of 2-(Phenethylthio)-1H-benzo[d] imidazole (ZR-1): A mixture of 2-mercaptobenzimidazole (1.50 g, 0.01 mol.), potassium hydroxide (0.56 g, 0.01 mol.), acetonitrile 50 ml has been heated and stirred under reflux pressure for 15 minutes until the temperature reach 50°C, the (2-bromoethyl) benzene 2-phenyl-ethyl (1.85 g, 0.01 mol.) was added drop wise, keeping the temperature between (40-50)°C, the reacted mixture was heated and stirred under reflux for 1.30 hours. After cooling, the precipitate filtered and washing with distilled water, white crystal was formed by recrystallization from ethanol-water, finally the product weighted and characterization by DSC, Infrared spectroscopy (FT-IR), 1H-NMR and 13C-NMR (Scheme 1).
SC: (160°C), FT-IR (KBr): C-H stretching Aromatic (2958.27 cm-1), C=C Stretching Aromatic (1496.49 cm-1), C-N Stretching Aromatic (1268.93 cm-1), C-S Stretching (593.97 cm-1), N-H Stretching (3434.60 cm-1) CH2 aliphatic (2786.63 cm-1), 1H-NMR: two types of CH2 proton (3-4 ppm, 3H, triplet), CH of aromatic (7.0 ppm-8.0 ppm), 13C-NMR: C11, C12 (35.228-37.74) ppm, C3, C6 (115.090-116.115) ppm, C1, C2 (123.640), C16 (128.088 ppm), C14, C18 (130.036 ppm), C15, C17 (130.036), C4, C5 (141.518 ppm), Elemental analysis: for C7H6N2S: Calcd: C, 70.83%; H, 5.55%; N, 11.01%. Found: C, 70.68%; H, 5.58%; N, 11.22%. MW 254.35.
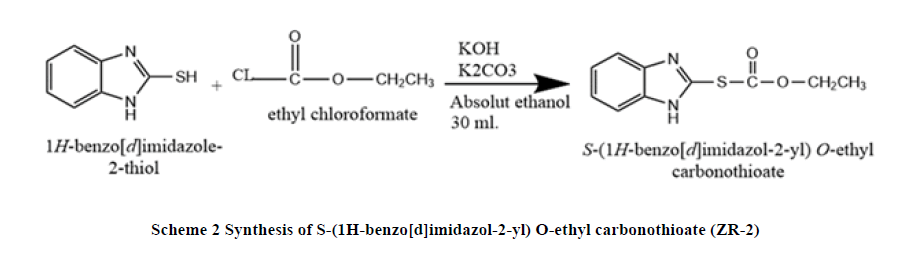
Synthesis of S-(1H-benzo[d]imidazol-2-yl) O-ethyl carbonothioate (ZR-2): A mixture of 2-mercaptobenzimidazole (1.50 g, 0.01 mol.), potassium hydroxide (0.56 g, 0.01 mol.), potassium carbonate (1.38 g, 0.01 mol.), In absolute ethanol 30 ml, heat and stirred under reflux pressure for 5 minutes until the temperature reach (30-40)°C, the ethyl chloroformate (1.08 g, 0.01 mol.) was added drop wise, the mixture was heated and stirred under reflux for 2 hours, after cooling, add water with ice to the mixture than solid white crystal precipitate, filtered, finally the product weighted and characterization by DSC, Inferred spectroscopy (FT-IR), 1H-NMR and C13 NMR (Scheme 2).
DSC: (160°C), FT-IR (KBr): C-H stretching Aromatic (2983.34cm-1), C=C Stretching Aromatic (1606.34 cm-1), C-N Stretching Aromatic (1322.93 cm-1), C-S Stretching (626.68 cm-1), N-H Stretching (3453.88 cm-1),1H-NMR: CH3 protons (1.38 ppm, 3H, triplet), CH2 protons (3.344 ppm, 2H, multiplet), Two types of aromatic proton (7.0 ppm-8.0 ppm), 13C-NMR: C15 (16.778 ppm), C14 (55.822 ppm), C3, C6 (106.966 ppm), C1, C2 (125.003ppm), C8 (146.163 ppm), C11 (163.527 ppm), Elemental analysis: for C10H10N2O2S: Calcd: C, 54.04%; H, 4.54%, N, 12.60%.
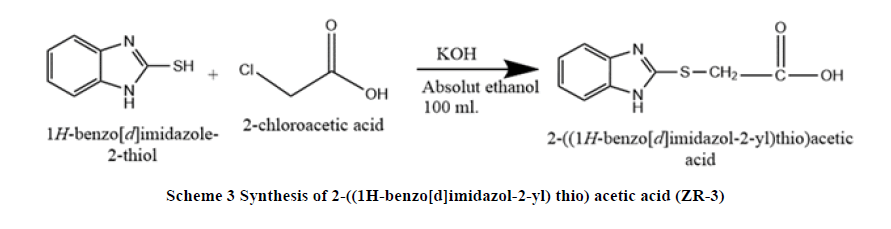
Synthesis of 2-((1H-benzo[d]imidazol-2-yl) thio) acetic acid (ZR-3): A mixture of 2-mercaptobenzimidazole (1.50 g, 0.03 mol.), potassium hydroxide (2 g, >0.03 mol.) in100 ml in absolute ethanol heat with stirring under reflux, then 2-chloroacetic acid (3.5g, >0.03 mol.) was added dropped wise with continues stirring and heat up to 60°C for 4 hours. The mixture was cooled to room temperature and added water then formed white foam on the surface, filtered and dry in oven at temperature 50°C for 30 minutes. The precipitate weighted and characterized by DSC, 1H NMR, 13C NMR, and FI-IR (Scheme 3).
DSC: (230°C), FT-IR (KBr): C-H Stretching Aromatic (2915.84cm-1), C=C Stretching Aromatic (1573.63 cm-1), C-S Stretching (605.54 cm-1), N-H Stretching (3430.74cm-1), 1H-NMR: CH2 proton (3.156 ppm, singlet), CH of aromatic (7-8) ppm, 13C-NMR: C11 (36.303), C6, C3 (115.622), C1, C2 (123.974), C4, C5 (140.101), C8 (163.527), C13 (172.934), Elemental analysis: for C9H8N2O2S: Calcd: C, 51.91%; H, 3.87%, N, 13.45%. Found: C, 52.02%, H, 3.75%; N, 13.53%. MW 208.03.
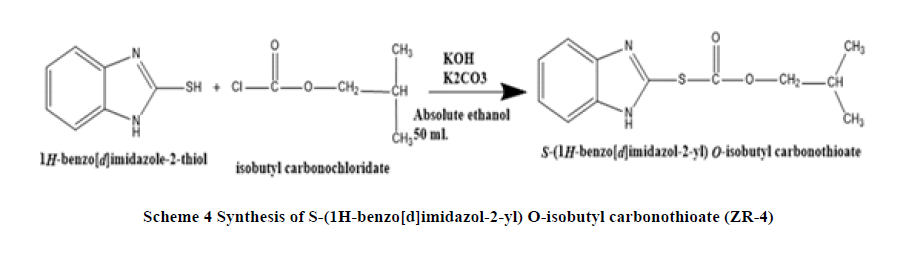
Synthesis of S-(1H-benzo[d]imidazol-2-yl) O-isobutyl carbonothioate (ZR-4): A mixture of 2-mercaptobenzimidazole (1.50199 g, 0.01 mol.), K2CO3 (1.38 g, 0.01 mol.), KOH (0.56 g, 0.01), in 50 ml absolute ethanol, heated and stirred for 45 minutes at 60°C. Cool to room temperature and add isobutyl chloroformate (0.01 mol, 1.36 ml) dropped wise the mixture heated to 45°C under reflex for 2 hours. The mixture cool to room temperature and poured in to 100 ml of iced water. Precipitate was filtered, recrystallized from ethanol-water. The greenish Product characterized by 1HNMR, 13C NMR, DSC, and FT-IR (Scheme 4).
FT-IR (KBr): C-H stretching Aromatic (2964.05 cm-1), C=C Stretching Aromatic (1621.84 cm-1), C-N Stretching Aromatic (1326.79 cm-1), C-S Stretching (628.68cm-1), N-H Stretching (3110.62 cm-1), Ester stretching (1737.55 cm-1), 1H-NMR: Two types CH3 protons (2.504 ppm, 6H, doublet), CH proton (3.251 ppm, 1H, multiplet), CH2 aliphatic (4.617 ppm, 2H, doublet), CH of aromatic protons (7.0 ppm-8.0 ppm, multiplet), 13C-NMR: C16, C17 (20.5 ppm), C15 (36.24 ppm), C14 (72.72 ppm), C3, C6 (109.418), C1, C2 (122.246), C4, C5 (130.990), C8 (159.19 ppm), C11 (165.93 ppm), Elemental analysis: for C9H8N2O2S : Calcd: C, 57.58%; H, 5.64 %; N, 11.19%, Found: C, 57.55%; H, 5.63%; N, 11.25%. MW 250.32.
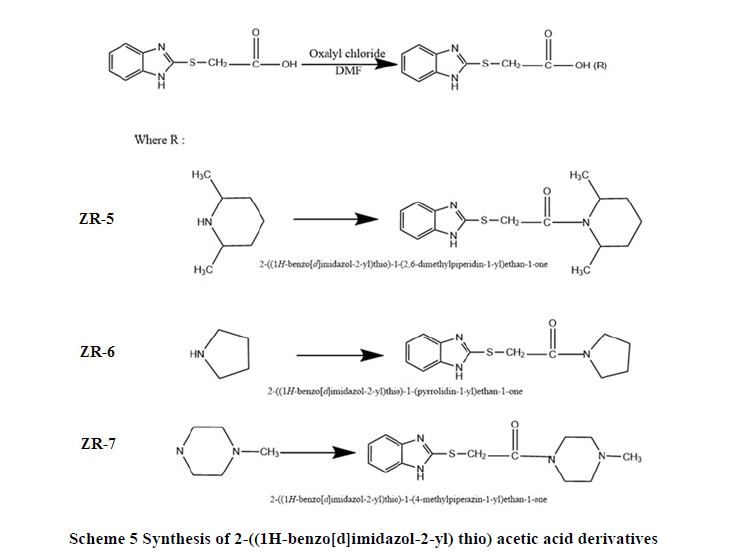
Synthesis of 2-((1H-benzo[d]imidazol-2-yl) thio) acetic acid derivatives (ZR-5, ZR-6, ZR-7): A mixture of (ZR- 3) (2.08 g, 0.01 mol.), oxalyl chloride (0.8 ml), few drops of dimethyl formamide, in dichloromethane (50 ml), has been heated and stirred under reflex for 18 hours at 25°C. Added appropriate amount of: 2, 6-dimethyl piperidine, Pyrrolidine, N-methyl piperazine drop wise and stirred at room temperature for 18 hours under reflex. The precipitate was filtered, wash with little amount of distilled water generate different colors, orange (ZR-5), green (ZR-6), orange (ZR-7). Different products confirm through 1H-NMR, 13C-NMR, DSC, and FT-IR (Scheme 5).
2-((1H-benzo[d]imidazol-2-yl) thio)-1-(2, 6-dimethylpiperidin-1-yl) ethan-1-one (ZR-5): DSC: (165-170)°C, FTIR (KBr): C-H stretching Aromatic (2965.98cm-1), C=C Stretching Aromatic (1508.06 cm-1), C-N Stretching Aromatic (1373.07 cm-1), C-S Stretching (698.11 cm-1), N-H Stretching (3133.76 cm-1), CH3 (2923.56) stretching aliphatic, 1H-NMR: Two types of CH3 protons (1.0 ppm-2.0 ppm, 3H, multiplet), various proton on cyclic amine (2.5 ppm- 3.5ppm, multiplet), two types of CH2 protons (3.75 ppm-4.0ppm, 2H, multiplet), CH2 proton (4.67ppm, 2H, S-CH2, singlet), CH of aromatic (7-8) ppm, 13C-NMR: C20, C21 (18.876), C17 (22.061), C16, C18 (29.560), C19, C15 (37.384-42.936), C11 (52.223), C6, C3 (111.796), C1, C2 (118.503), C5, C4 (123.421), C8 (149.287), C12 (164.300), Elemental analysis: for C16H21N3OS : Calcd: C, 63.34%; H; 6.98%; N, 13.85%, Found: C, 63.55%, H, 6.95%, N, 13.55%. M.wt = 303.42.
2-((1H-benzo[d]imidazol-2-yl) thio)-1-(pyrrolidin-1-yl) ethan-1-one (ZR-6): FT-IR (KBr): C-H stretching Aromatic (3000 cm-1), C=C Stretching Aromatic (1712.48 cm-1), C-N Stretching Aromatic (1330.64 cm-1), C-S Stretching (644.11cm-1), N-H Stretching (3100.0 cm-1), Ketone stretching (1754.90 cm-1),1H-NMR: Various proton of cyclic amine (2.5ppm- 4.0 ppm, multiplet), CH2 aliphatic (4.75ppm, 2H, singlet), CH of aromatic (7-8 ppm) (Figure 3.6)., 13C-NMR: C15, C16 (23.108), C18 (34.661), C14, C17 (39.467), C14, C17 (45.021), C3, C6 (11.801), C1, C2 (113.198), C4, C5 (118.494), C8 (149.919), C11 (168.743), Elemental analysis: for C13H15N3OS: Calcd: C, 59.75%; H, 5.79%; N, 16.08%. M.wt = 261.34.
2-((1H-benzo[d]imidazol-2-yl) thio)-1-(4-methylpiperazin-1-yl) ethan-1-one (ZR-7): FT-IR (KBr): C-H stretching Aromatic (2923.56 cm-1), C=C Stretching Aromatic (1500.06 cm-1), C-N Stretching Aromatic (1303.64 cm-1), C-S Stretching (698.11cm-1), N-H Stretching (3396.03 cm-1), Ketone stretching (1739.48 cm-1), C-N tertiary amine(1265.07 cm-1), CH3 Stretching (3000.0 cm-1),1H-NMR: Various protons of cyclic methyl piperazine (2.0 ppm – 4.0 ppm, multiplet), CH2 aliphatic (4.75ppm, 2H, singlet), CH of aromatic (7.0 ppm-8.0 ppm, multiplet), 13CNMR: C11 (40.026), C20 (42.360), C15, C9 (49.130), C18, C16 (51.870), C3, C6 (113.209), C1, C2 (118.499), C4, C5 (123.957), C8 (129.743), C13 (165.937), Elemental analysis: for C14H18N4OS: Calcd: C, 57.91%; N, 6.25 %; N, 19.29%. M.wt = 290.39.
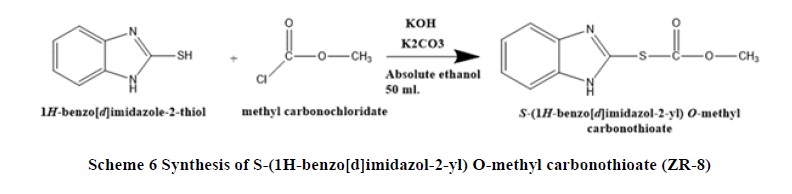
Synthesis of S-(1H-benzo[d]imidazol-2-yl) O-methyl carbonothioate (ZR-8): A mixture of 2-mercaptobenzimidazole (1.50 g, 0.01 mol.), potassium hydroxide (0.56 g, 0.01 mol.), potassium carbonate (1.38 g, 0.01 mol.), Absolute ethanol 50 ml. Heated and stirred for 30 minutes until the temperature reach 50°C, the methyl chloroformate (0.945 g, 0.01 mol.) was added drop wise. Keeping the temperature between (40-50)°C for 30 minutes. The reaction was cooled to room temperature and the white mixture filtrated and concentrated under reduces pressure. White crystals formed, recrystallized in ethanol-water. The product weighted and the structure was confirmed by DSC, Inferred spectroscopy (FT-IR), 1H-NMR and 13C-NMR (Scheme 6).
S-(1H-benzo[d]imidazol-2-yl) O-methyl carbonothioate (ZR-8): FT-IR (KBr): C-H stretching Aromatic (3100.0 cm-1), C=C Stretching Aromatic (1617.98 cm-1), C-N Stretching Aromatic (1330.64 cm-1), C-S Stretching (761.74- 599.75)cm-1, N-H Stretching (3149.19 cm-1), Thioester stretching (1733.69 cm-1), 1H-NMR: CH3 proton (2.5ppm, 3H, singlet), CH of aromatic (7-8) ppm, 13C-NMR: C14 (39.490), C3, C6 (109.723), C1, C12 (119.004), C4, C5 (149.267), C8 (165.936), Elemental analysis: for C9H8N2O2S; Calcd: C, 51.91%; H, 3.87%; N, 13.45%. M.WT=208.03 g/mol.
Conclusion
The approach for this synthesize derivatives of 2-mercaptobenzimidazole and elucidation by NMR, FT-IR of their structures was consistent with the design compounds. The compound ZR-5 (2-((1H-benzo[d]imidazol-2-yl) thio)-1- (2, 6-dimethylpiperidin-1-yl) ethan-1-one) its shows higher affinity for DHFR. Compound (ZR-8) showed excellent antifungal activity in comparison with miconazole and good antibacterial activity compared to ciprofloxacin and trimethoprim.
Declarations
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgment
The authors would like to thank the university of Petra/faculty of pharmacy for providing the necessary facilities to carry out this work.
References
- Turkey, R. H., and Ammar Abdul Razzak M. Kubba. "Synthesis, characterization and antibacterial activity of new 5-ethoxy-2-mercapto benzimidazole derivatives." Journal of Pharmacy Research, Vol. 10, No. 12, 2016, pp. 814-24.
- Walia, R. et al. "Benzimidazole derivatives- an overview." International Journal of Research in Pharmacy and Chemistry, Vol. 1, No. 3, 2011, pp. 565-74.
- Soni, B. (2014) "A short review on potential activities of benzimidazole derivatives." PharmaTutor, Vol. 2, No. 8, 2014, pp. 110-8.
- Soderlind, K. J. et al. "Bis-benzimidazole anticancer agents: targeting human tumour helicases." Anticancer Drug Design, Vol. 14, No. 1, 1999, pp. 19-36.
- Kumar, K. et al. "Benzimidazole-based antibacterial agents against Francisella tularensis." Bioorganic and Medicinal Chemistry, Vol. 21, No. 11, 2013, pp. 3318-26.
- Ke, Yazhen, et al. "Combinatorial synthesis of benzimidazole-azo-phenol derivatives as antifungal agents." Combinatorial Chemistry & High Throughput Screening, Vol. 17, No. 1, 2014, pp. 89-95.
- Tonelli, M. et al. "Antiviral activity of benzimidazole derivatives. 1. Antiviral activity of 1-substituted-2-[(Benzotriazol-1/2-yl)methyl]benzimidazoles." Chemistry & Biodiversity, Vol. 5, No. 11, 2008, pp. 2386-401.
- Shingalapur, R. V. et al. "Derivatives of benzimidazole pharmacophore: Synthesis, anticonvulsant, antidiabetic and DNA cleavage studies." European Journal of Medicinal Chemistry, Vol. 45, No. 5, 2010, pp. 1753-9.
- A Datar, P., and Saleel A Limaye. "Design and synthesis of Mannich bases as benzimidazole derivatives as analgesic agents." Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents), Vol. 14 No. 1, 2015, pp. 35-46.
- Achar, K. C. S., Kallappa M. Hosamani, and Harisha R. Seetharamareddy. "In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives." European Journal of Medicinal Chemistry, Vol. 45, No. 5, 2010, pp. 2048-54.
- Bansal, Y., and Om Silakari. "The therapeutic journey of benzimidazoles: a review." Bioorganic & Medicinal Chemistry, Vol. 20, No. 21, 2012, pp. 6208-36.
- Gurvinder, S., Maninderjit, K. and Mohan, C. "Benzimidazole: The latest information of biological activities." International Research Journal of Pharmacy, Vol. 4, No. 1, 2013, pp. 82-7.
- Khalafi-Nezhad, A. et al. "Design, synthesis, antibacterial and QSAR studies of benzimidazole and imidazole chloroaryloxyalkyl derivatives." Bioorganic and Medicinal Chemistry, Vol. 13, No.6, 2005, pp. 1931-8.
- Verma, A., Sunil Joshi and Deepika Singh. "ChemInform abstract: imidazole: having versatile biological activities." ChemInform, Vol. 45, No. 44, 2014.
- Gorepatil, P. B. et al. "One pot synthesis of antimicrobial active new 2-benzimidazolesulfonamide derivatives from 2-mercaptobenzimidazole " Journal Current Chemical and Pharmaceutical Sciences, Vol. 2, No. 4, 2012, pp. 367-74.
- Jabali J. et al., "Synthesis and Spectral Studies of 2-mercapto-5-methoxy-1 H -benzimidazole: An Imperative Medicinal Intermediate." Der Chemica Sinica, Vol. 3, No. 1, 2012, pp. 76-9.
- Mahmoodi, Nosrat O., and Shahryar Ghodsi. "Thiazolyl-pyrazole-biscoumarin synthesis and evaluation of their antibacterial and antioxidant activities." Research on Chemical Intermediates, Vol. 43, No.2, 2017, pp. 661-78.
- Anandarajagopal, K., et al. "Synthesis and characterization of 2-mercaptobenzimidazole derivatives as potential analgesic agents." Journal of Chemical and Pharmaceutical Research, Vol. 2, No.3, 2010, pp. 230.
- Ahamed, Mohammed R., Shetha F. Narren, and Amal S. Sadiq. "Synthesis of 2-mercaptobenzimidazole and some of its derivatives." Al-Nahrain Journal of Science, Vol. 16, No.2, 2013, pp. 77-83.
- Ouaket, Amine, et al. "DPPH scavenging activity of some Bis-benzimidazole derivatives." Mediterranean Journal of Chemistry, Vol. 8, No. 2, 2019, pp. 103-7.
- Ravesh, A. and Devi, S. "Synthesis, characterization and antimicrobial activities of organosilicon(IV) complexes." Asian Journal of Chemistry, Vol. 30, No. 8, 2018, pp. 1811-4.
- Balouiri, Mounyr, Moulay Sadiki, and Saad Koraichi Ibnsouda. "Methods for in vitro evaluating antimicrobial activity: A review." Journal of Pharmaceutical Analysis, Vol. 6, No. 2, 2016, pp. 71-9.
- More, G., et al. "Antimicrobial activity of medicinal plants against oral microorganisms." Journal of Ethnopharmacology, Vol. 119, No. 3, 2008, pp. 473-7.
- NCCLS. National committee for clinical laboratory standards for antimicrobial disk susceptibility testing (M02-A11) Wayne, PA: NCCL; 2012. pp. 32.
- Khodarahmi, G. A., et al. "Antibacterial, antifungal and cytotoxic evaluation of some new 2, 3-disubstituted 4 (3H)-quinazolinone derivatives." Research in Pharmaceutical Sciences, Vol. 7, No. 3, 2012, pp. 151-8.
- Smith, John A., et al. "Comparison of agar disk diffusion, microdilution broth, and agar dilution for testing antimicrobial susceptibility of coagulase-negative staphylococci." Journal of Clinical Microbiology, Vol. 25, No. 9, 1987, pp. 1741-6.
- Alkhafaji, Alaa, et al. "Synthesis, structural elucidation, and evaluation of antimicrobial activity of 5-ethoxy-2-mercaptobenzimidazole derivatives." International Journal of Medical Research & Health Sciences, Vol. 7, No. 5, 2018, pp. 117-27.
- Defrenza, Ivana, et al. "1, 3-Benzothiazoles as antimicrobial agents." Journal of Heterocyclic Chemistry, Vol. 52, No. 6, 2015, pp. 1705-12.